Topical Imiquimod for the Treatment of High-Grade Squamous Intraepithelial Lesions of the Cervix: A Randomized Controlled Trial
- PMID: 33957649
- PMCID: PMC8132915
- DOI: 10.1097/AOG.0000000000004384
Topical Imiquimod for the Treatment of High-Grade Squamous Intraepithelial Lesions of the Cervix: A Randomized Controlled Trial
Abstract
Objective: To evaluate the histologic response rate of high-grade squamous intraepithelial lesions (HSIL) of the cervix after topical application of 5% imiquimod cream.
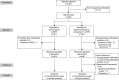
Methods: In this phase II trial, women with cervical HSIL (cervical intraepithelial neoplasia [CIN] 2-3) were randomly assigned to 250 mg of 5% imiquimod cream applied to the cervix weekly for 12 weeks, followed by loop electrosurgical excision procedure (LEEP) without preceding treatment. The sample size was calculated based on the HSIL regression rates previously reported by Grimm et al. The primary outcome was rate of histologic regression (to CIN 1 or less) in LEEP specimens. Prespecified secondary endpoints included surgical margin status and adverse events. Outcomes were stratified by human papillomavirus type and lesion grade (CIN 2 or CIN 3). Results were reported according to per protocol (PP) and intention-to-treat (ITT) analyses.
Results: Ninety women were enrolled: 49 in the experimental group and 41 in the control group. In the PP population, histologic regression was observed in 23 of 38 participants (61%) in the experimental group compared with 9 of 40 (23%) in the control group (P=.001). Surgical margins were negative for HSIL in 36 of 38 participants (95%) in the experimental group and 28 of 40 (70%) in the control group (P=.004). In the ITT population, rates of histologic regression also were significantly higher in the experimental group. Rates of adverse events in the experimental group were 74% (28/38) in the PP population and 78% (35/45) in the ITT population. Adverse events were mild, with abdominal pain being the most common. Three patients in the experimental group had grade 2 adverse events, including vaginal ulcer, vaginal pruritus with local edema, and moderate pelvic pain.
Conclusion: Weekly topical treatment with imiquimod is effective in promoting regression of cervical HSIL.
Clinical trial registration: ClinicalTrials.gov, NCT03233412.
Copyright © 2021 The Authors. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Financial Disclosure Júlio C. Possati-Resende disclosed they received money from Becton, Dickinson, and Company. The other authors did not report any potential conflicts of interest.
Figures



References
-
- Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. . Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ 2008;337:a1284. doi: 10.1136/bmj.a1284.PMID:18801868 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

