TORC1 regulates the transcriptional response to glucose and developmental cycle via the Tap42-Sit4-Rrd1/2 pathway in Saccharomyces cerevisiae
- PMID: 33957926
- PMCID: PMC8103650
- DOI: 10.1186/s12915-021-01030-3
TORC1 regulates the transcriptional response to glucose and developmental cycle via the Tap42-Sit4-Rrd1/2 pathway in Saccharomyces cerevisiae
Abstract
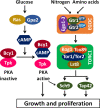
Background: Target of Rapamycin Complex 1 (TORC1) is a highly conserved eukaryotic protein complex that couples the presence of growth factors and nutrients in the environment with cellular proliferation. TORC1 is primarily implicated in linking amino acid levels with cellular growth in yeast and mammals. Although glucose deprivation has been shown to cause TORC1 inactivation in yeast, the precise role of TORC1 in glucose signaling and the underlying mechanisms remain unclear.
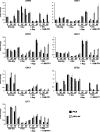
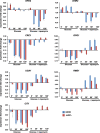
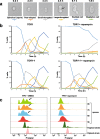
Results: We demonstrate that the presence of glucose in the growth medium is both necessary and sufficient for TORC1 activation. TORC1 activity increases upon addition of glucose to yeast cells growing in a non-fermentable carbon source. Conversely, shifting yeast cells from glucose to a non-fermentable carbon source reduces TORC1 activity. Analysis of transcriptomic data revealed that glucose and TORC1 co-regulate about 27% (1668/6004) of yeast genes. We demonstrate that TORC1 orchestrates the expression of glucose-responsive genes mainly via the Tap42-Sit4-Rrd1/2 pathway. To confirm TORC1's function in glucose signaling, we tested its role in spore germination, a glucose-dependent developmental state transition in yeast. TORC1 regulates the glucose-responsive genes during spore germination and inhibition of TORC1 blocks spore germination.
Conclusions: Our studies indicate that a regulatory loop that involves activation of TORC1 by glucose and regulation of glucose-responsive genes by TORC1, mediates nutritional control of growth and development in yeast.
Keywords: Spore germination; TORC1; Tap42/Sit4/Rrd1-2 module; Transcriptional response to glucose.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

