Comparison of a mycobacterial phage assay to detect viable Mycobacterium avium subspecies paratuberculosis with standard diagnostic modalities in cattle with naturally infected Johne disease
- PMID: 33957980
- PMCID: PMC8103604
- DOI: 10.1186/s13099-021-00425-5
Comparison of a mycobacterial phage assay to detect viable Mycobacterium avium subspecies paratuberculosis with standard diagnostic modalities in cattle with naturally infected Johne disease
Abstract
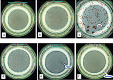
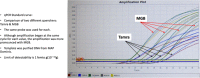
Background: Mycobacterium avium subspecies paratuberculosis (MAP), the cause of Johne disease, is a slow growing mycobacterium. Viable MAP detection is difficult, inconstant and time-consuming. The purpose of this study was to compare a rapid phage/qPCR assay performed on peripheral blood mononuclear cells (PBMCs) with three standard methods of MAP detection: fecal MAP PCR; plasma antigen-specific IFN-γ & serum MAP ELISA hypothesizing that, if sensitive and specific, Johne animals would be positive and Control animals negative. We studied a well characterized herd of Holstein cattle that were naturally infected with MAP and their Controls.
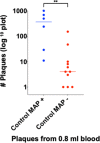
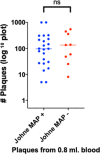
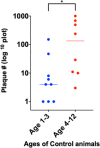
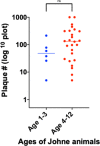
Results: With phage/qPCR 72% (23/32) of Johne and 35% (6/17) of Controls were MAP positive. With fecal PCR 75% (24/32) of Johne and 0% (0/17) of Controls were MAP positive. With plasma antigen-specific IFN-γ 69% (22/32) of Johne and 12% (2/17) of Controls were MAP positive. With serum MAP ELISA, 31% (10/32) of Johne and 0% (0/17) of Controls were MAP positive. When phage / qPCR and fecal PCR results were combined, 100% (32/32) Johne and 35% (6/17) of Control animals were MAP positive. Younger Control animals (1-3 years) had significantly fewer plaques (25 ± 17 SEM) than older Controls (4-12 years) (309 ± 134 p = 0.04). The same trend was not observed in the Johne animals (p = 0.19).
Conclusions: In contrast to our hypothesis, using the phage/qPCR assay we find that viable circulating MAP can rapidly be detected from the blood of animals infected with, as well as those in the Control group evidently colonized by MAP. These data indicate that the presence of viable MAP in blood does not necessarily signify that an animal must of necessity be demonstrably ill or be MAP positive by standard diagnostic methods.
Keywords: Crohn disease; Johne disease; Mycobacteriophage; Mycobacterium avium subspecies paratuberculosis (MAP); Peripheral blood mononuclear cells (PBMCs); Phage assay; Quantitative PCR.
Conflict of interest statement
Dr. Greenstein is an Associate Editor of
Figures

Similar articles
-
Evaluation of the limitations and methods to improve rapid phage-based detection of viable Mycobacterium avium subsp. paratuberculosis in the blood of experimentally infected cattle.BMC Vet Res. 2016 Jun 16;12(1):115. doi: 10.1186/s12917-016-0728-2. BMC Vet Res. 2016. PMID: 27305900 Free PMC article.
-
Sensitive and specific detection of viable Mycobacterium avium subsp. paratuberculosis in raw milk by the peptide-mediated magnetic separation-phage assay.J Appl Microbiol. 2017 May;122(5):1357-1367. doi: 10.1111/jam.13425. Epub 2017 Mar 27. J Appl Microbiol. 2017. PMID: 28235155
-
Comparison of fecal pooling methods and DNA extraction kits for the detection of Mycobacterium avium subspecies paratuberculosis.Microbiologyopen. 2016 Feb;5(1):134-42. doi: 10.1002/mbo3.318. Epub 2015 Dec 15. Microbiologyopen. 2016. PMID: 26666871 Free PMC article.
-
Long-term detection of Mycobacterium avium subspecies paratuberculosis in individual and bulk tank milk from a dairy herd with a low prevalence of Johne's disease.J Dairy Sci. 2013 Jun;96(6):3517-24. doi: 10.3168/jds.2012-6466. Epub 2013 Apr 19. J Dairy Sci. 2013. PMID: 23608495
-
Bacteriophage-Based Methods for Detection of Viable Mycobacterium avium subsp. paratuberculosis and Their Potential for Diagnosis of Johne's Disease.Front Vet Sci. 2021 Mar 11;8:632498. doi: 10.3389/fvets.2021.632498. eCollection 2021. Front Vet Sci. 2021. PMID: 33778037 Free PMC article. Review.
Cited by
-
Human antibodies against Mycobacterium avium ssp. paratuberculosis combined with cytokine levels for the diagnosis and selection of Crohn's disease patients for anti-mycobacterial therapy-A pilot study.PLoS One. 2024 Oct 4;19(10):e0308911. doi: 10.1371/journal.pone.0308911. eCollection 2024. PLoS One. 2024. PMID: 39365776 Free PMC article.
-
The evidence for Mycobacterium avium subspecies paratuberculosis (MAP) as a cause of nonsolar uveal melanoma: a narrative review.Transl Cancer Res. 2023 Feb 28;12(2):398-412. doi: 10.21037/tcr-22-2540. Epub 2022 Dec 16. Transl Cancer Res. 2023. PMID: 36915598 Free PMC article. Review.
-
Cytokine expression in subjects with Mycobacterium avium ssp. paratuberculosis positive blood cultures and a meta-analysis of cytokine expression in Crohn's disease.Front Cell Infect Microbiol. 2024 Feb 13;14:1327969. doi: 10.3389/fcimb.2024.1327969. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38415011 Free PMC article.
-
A Review on Mycobacteriophages: From Classification to Applications.Pathogens. 2022 Jul 7;11(7):777. doi: 10.3390/pathogens11070777. Pathogens. 2022. PMID: 35890022 Free PMC article. Review.
References
-
- Johne HA, Frothingham L. Ein eigenthumlicher fall von tuberculose beim rind (A particular case of tuberculosis in a cow) Dtsch Zeitschr Tiermed, Vergl Pathol. 1895;21:438–454.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

