Prevalence of SARS-CoV-2 Antibodies in a Multistate Academic Medical Center
- PMID: 33958053
- PMCID: PMC7997730
- DOI: 10.1016/j.mayocp.2021.03.015
Prevalence of SARS-CoV-2 Antibodies in a Multistate Academic Medical Center
Abstract
Objective: To estimate the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in health care personnel.
Methods: The Mayo Clinic Serology Screening Program was created to provide a voluntary, two-stage testing program for SARS-CoV-2 antibodies to health care personnel. The first stage used a dried blood spot screening test initiated on June 15, 2020. Those participants identified as reactive were advised to have confirmatory testing via a venipuncture. Venipuncture results through August 8, 2020, were considered. Consent and authorization for testing was required to participate in the screening program. This report, which was conducted under an institutional review board-approved protocol, only includes employees who have further authorized their records for use in research.
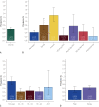
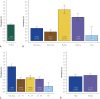
Results: A total of 81,113 health care personnel were eligible for the program, and of these 29,606 participated in the screening program. A total of 4284 (14.5%) of the dried blood spot test results were "reactive" and warranted confirmatory testing. Confirmatory testing was completed on 4094 (95.6%) of the screen reactive with an overall seroprevalence rate of 0.60% (95% CI, 0.52% to 0.69%). Significant variation in seroprevalence was observed by region of the country and age group.
Conclusion: The seroprevalence for SARS-CoV-2 antibodies through August 8, 2020, was found to be lower than previously reported in other health care organizations. There was an observation that seroprevalence may be associated with community disease burden.
Copyright © 2021 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- New York Times public repository. https://github.com/nytimes/covid-19-data
-
- USA Facts Our nation, in numbers. https://usafacts.org/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

