A Framework for Outpatient Infusion of Antispike Monoclonal Antibodies to High-Risk Patients with Mild-to-Moderate Coronavirus Disease-19: The Mayo Clinic Model
- PMID: 33958056
- PMCID: PMC7942148
- DOI: 10.1016/j.mayocp.2021.03.010
A Framework for Outpatient Infusion of Antispike Monoclonal Antibodies to High-Risk Patients with Mild-to-Moderate Coronavirus Disease-19: The Mayo Clinic Model
Abstract
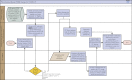
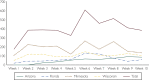
The administration of spike monoclonal antibody treatment to patients with mild to moderate COVID-19 is very challenging. This article summarizes essential components and processes in establishing an effective spike monoclonal antibody infusion program. Rapid identification of a dedicated physical infrastructure was essential to circumvent the logistical challenges of caring for infectious patients while maintaining compliance with regulations and ensuring the safety of our personnel and other patients. Our partnerships and collaborations among multiple different specialties and disciplines enabled contributions from personnel with specific expertise in medicine, nursing, pharmacy, infection prevention and control, electronic health record (EHR) informatics, compliance, legal, medical ethics, engineering, administration, and other critical areas. Clear communication and a culture in which all roles are welcomed at the planning and operational tables are critical to the rapid development and refinement needed to adapt and thrive in providing this time-sensitive beneficial therapy. Our partnerships with leaders and providers outside our institutions, including those who care for underserved populations, have promoted equity in the access of monoclonal antibodies in our regions. Strong support from institutional leadership facilitated expedited action when needed, from a physical, personnel, and system infrastructure standpoint. Our ongoing real-time assessment and monitoring of our clinical program allowed us to improve and optimize our processes to ensure that the needs of our patients with COVID-19 in the outpatient setting are met.
Copyright © 2021. Published by Elsevier Inc.
Figures

References
-
- Gulick R., Lance H., Masur H. National Institutes of Health; Bethesda, MD: 2021. COVID-19 treatment guidelines. NIH Vol 20212021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

