Smyd1 is essential for myosin expression and sarcomere organization in craniofacial, extraocular, and cardiac muscles
- PMID: 33958316
- PMCID: PMC9234968
- DOI: 10.1016/j.jgg.2021.03.004
Smyd1 is essential for myosin expression and sarcomere organization in craniofacial, extraocular, and cardiac muscles
Abstract
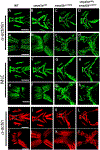
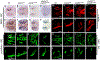
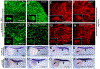
Skeletal and cardiac muscles are striated myofibers that contain highly organized sarcomeres for muscle contraction. Recent studies revealed that Smyd1, a lysine methyltransferase, plays a key role in sarcomere assembly in heart and trunk skeletal muscles. However, Smyd1 expression and function in craniofacial muscles are not known. Here, we analyze the developmental expression and function of two smyd1 paralogous genes, smyd1a and smyd1b, in craniofacial and cardiac muscles of zebrafish embryos. Our data show that loss of smyd1a (smyd1amb5) or smyd1b (smyd1bsa15678) has no visible effects on myogenic commitment and expression of myod and myosin heavy-chain mRNA transcripts in craniofacial muscles. However, myosin heavy-chain protein accumulation and sarcomere organization are dramatically reduced in smyd1bsa15678 single mutant, and almost completely diminish in smyd1amb5; smyd1bsa15678 double mutant, but not in smyd1amb5 mutant. Similar defects are also observed in cardiac muscles of smyd1bsa15678 mutant. Defective craniofacial and cardiac muscle formation is associated with an upregulation of hsp90α1 and unc45b mRNA expression in smyd1bsa15678 and smyd1amb5; smyd1bsa15678 mutants. Together, our studies indicate that Smyd1b, but not Smyd1a, plays a key role in myosin heavy-chain protein expression and sarcomere organization in craniofacial and cardiac muscles. Loss of smyd1b results in muscle-specific stress response.
Keywords: Cardiac muscle; Craniofacial muscle; Myosin; Sarcomere; Smyd1.
Copyright © 2021 Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, and Genetics Society of China. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest There is no conflict of interest.
Figures



Similar articles
-
Loss of zebrafish Smyd1a interferes with myofibrillar integrity without triggering the misfolded myosin response.Biochem Biophys Res Commun. 2018 Feb 5;496(2):339-345. doi: 10.1016/j.bbrc.2018.01.060. Epub 2018 Jan 10. Biochem Biophys Res Commun. 2018. PMID: 29331378
-
Defective sarcomere assembly in smyd1a and smyd1b zebrafish mutants.FASEB J. 2019 May;33(5):6209-6225. doi: 10.1096/fj.201801578R. Epub 2019 Feb 28. FASEB J. 2019. PMID: 30817176 Free PMC article.
-
Smyd1b is required for skeletal and cardiac muscle function in zebrafish.Mol Biol Cell. 2013 Nov;24(22):3511-21. doi: 10.1091/mbc.E13-06-0352. Epub 2013 Sep 25. Mol Biol Cell. 2013. PMID: 24068325 Free PMC article.
-
SMYD proteins: key regulators in skeletal and cardiac muscle development and function.Anat Rec (Hoboken). 2014 Sep;297(9):1650-62. doi: 10.1002/ar.22972. Anat Rec (Hoboken). 2014. PMID: 25125178 Review.
-
Developmental, Physiological and Phylogenetic Perspectives on the Expression and Regulation of Myosin Heavy Chains in Craniofacial Muscles.Int J Mol Sci. 2024 Apr 21;25(8):4546. doi: 10.3390/ijms25084546. Int J Mol Sci. 2024. PMID: 38674131 Free PMC article. Review.
Cited by
-
Lysine Methyltransferase SMYD1 Regulates Myogenesis via skNAC Methylation.Cells. 2023 Jun 22;12(13):1695. doi: 10.3390/cells12131695. Cells. 2023. PMID: 37443729 Free PMC article.
-
Hsf1 is essential for proteotoxic stress response in smyd1b-deficient embryos and fish survival under heat shock.FASEB J. 2025 Jan 15;39(1):e70283. doi: 10.1096/fj.202401875R. FASEB J. 2025. PMID: 39760245
-
Gene expression and functional analysis of Aha1a and Aha1b in stress response in zebrafish.Comp Biochem Physiol B Biochem Mol Biol. 2022 Oct-Dec;262:110777. doi: 10.1016/j.cbpb.2022.110777. Epub 2022 Jul 10. Comp Biochem Physiol B Biochem Mol Biol. 2022. PMID: 35830921 Free PMC article.
-
Expression of Smyd1b_tv1 by Alternative Splicing in Cardiac Muscle is Critical for Sarcomere Organization in Cardiomyocytes and Heart Function.Mol Cell Biol. 2024;44(12):543-561. doi: 10.1080/10985549.2024.2402660. Epub 2024 Sep 25. Mol Cell Biol. 2024. PMID: 39320962
-
Loss of Myomixer Results in Defective Myoblast Fusion, Impaired Muscle Growth, and Severe Myopathy in Zebrafish.Mar Biotechnol (NY). 2022 Oct;24(5):1023-1038. doi: 10.1007/s10126-022-10159-3. Epub 2022 Sep 9. Mar Biotechnol (NY). 2022. PMID: 36083384 Free PMC article.
References
-
- Berkholz J, Eberle R, Boller K, Munz B, 2018. siRNA-mediated inhibition of skNAC and Smyd1 expression disrupts myofibril organization: immunofluorescence and electron microscopy study in C2C12 cells. Micron 108, 6–10. - PubMed
-
- Busch-Nentwich E, Kettleborough R, Dooley CM, Scahill C, Sealy I, White R, Herd C, Mehroke S, Wali N, Carruthers S, et al., 2013. Sanger Institute zebrafish mutation project mutant data submission. ZFIN Direct Data Submission. https://zfin.org/ZDB-FISH-200210-2.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

