SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases
- PMID: 33958324
- PMCID: PMC8103562
- DOI: 10.1136/annrheumdis-2021-220461
SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases
Abstract
Objectives: To better understand the factors that influence the humoral immune response to vaccination against SARS-CoV-2 in patients with immune-mediated inflammatory diseases (IMIDs).
Methods: Patients and controls from a large COVID-19 study, with (1) no previous history of COVID-19, (2) negative baseline anti-SARS-CoV-2 IgG test and (3) SARS-CoV-2 vaccination at least 10 days before serum collection were measured for anti-SARS-CoV-2 IgG. Demographic, disease-specific and vaccination-specific data were recorded.
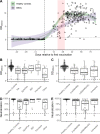
Results: Vaccination responses from 84 patients with IMID and 182 controls were analysed. While all controls developed anti-SARS-CoV-2 IgG, five patients with IMID failed to develop a response (p=0.003). Moreover, 99.5% of controls but only 90.5% of patients with IMID developed neutralising antibody activity (p=0.0008). Overall responses were delayed and reduced in patients (mean (SD): 6.47 (3.14)) compared with controls (9.36 (1.85); p<0.001). Estimated marginal means (95% CI) adjusted for age, sex and time from first vaccination to sampling were 8.48 (8.12-8.85) for controls and 6.90 (6.45-7.35) for IMIDs. Significantly reduced vaccination responses pertained to untreated, conventionally and anticytokine treated patients with IMID.
Conclusions: Immune responses against the SARS-CoV-2 are delayed and reduced in patients with IMID. This effect is based on the disease itself rather than concomitant treatment.
Keywords: COVID-19; biological therapy; epidemiology; vaccination.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity.Ann Rheum Dis. 2021 Oct;80(10):1345-1350. doi: 10.1136/annrheumdis-2021-220781. Epub 2021 Jul 20. Ann Rheum Dis. 2021. PMID: 34285048
-
Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2.Ann Rheum Dis. 2021 Oct;80(10):1317-1321. doi: 10.1136/annrheumdis-2021-220503. Epub 2021 Jun 18. Ann Rheum Dis. 2021. PMID: 34144967
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
-
Risk and prognosis of SARS-CoV-2 infection and vaccination against SARS-CoV-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations.Ann Rheum Dis. 2022 Mar;81(3):422-432. doi: 10.1136/annrheumdis-2021-221575. Epub 2021 Dec 7. Ann Rheum Dis. 2022. PMID: 34876462
-
Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases.Ann Rheum Dis. 2021 Oct;80(10):1255-1265. doi: 10.1136/annrheumdis-2021-221244. Epub 2021 Sep 7. Ann Rheum Dis. 2021. PMID: 34493491 Free PMC article. Review.
Cited by
-
Immunogenicity and Safety of mRNA Anti-SARS-CoV-2 Vaccines in Patients with Systemic Lupus Erythematosus.Vaccines (Basel). 2022 Jul 30;10(8):1221. doi: 10.3390/vaccines10081221. Vaccines (Basel). 2022. PMID: 36016108 Free PMC article.
-
Safety of COVID-19 Vaccine in Patients with Rheumatic and Musculoskeletal Diseases.Mediterr J Rheumatol. 2024 Feb 8;35(1):123-133. doi: 10.31138/mjr.140223.sof. eCollection 2024 Mar. Mediterr J Rheumatol. 2024. PMID: 38736958 Free PMC article.
-
Antibody Response to BNT162b2 Vaccine in Immune Modifiers-Treated Psoriatic Patients.J Psoriasis Psoriatic Arthritis. 2022 Jan;7(1):24-28. doi: 10.1177/24755303211056059. Epub 2021 Nov 12. J Psoriasis Psoriatic Arthritis. 2022. PMID: 39296727 Free PMC article.
-
Effectiveness and waning of protection with the BNT162b2 vaccine against the SARS-CoV-2 Delta variant in immunocompromised individuals.Front Immunol. 2023 Nov 2;14:1247129. doi: 10.3389/fimmu.2023.1247129. eCollection 2023. Front Immunol. 2023. PMID: 38022626 Free PMC article.
-
Vaccination for SARS-CoV-2 in Patients With Psoriatic Arthritis: Can Therapy Affect the Immunological Response?Front Med (Lausanne). 2022 Feb 28;9:811829. doi: 10.3389/fmed.2022.811829. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35295608 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

