Continuing versus withdrawing ixekizumab treatment in patients with axial spondyloarthritis who achieved remission: efficacy and safety results from a placebo-controlled, randomised withdrawal study (COAST-Y)
- PMID: 33958326
- PMCID: PMC8292566
- DOI: 10.1136/annrheumdis-2020-219717
Continuing versus withdrawing ixekizumab treatment in patients with axial spondyloarthritis who achieved remission: efficacy and safety results from a placebo-controlled, randomised withdrawal study (COAST-Y)
Abstract
Objectives: The objective of COAST-Y was to evaluate the effect of continuing versus withdrawing ixekizumab (IXE) in patients with axial spondyloarthritis (axSpA) who had achieved remission.
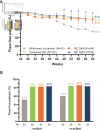
Methods: COAST-Y is an ongoing, phase III, long-term extension study that included a double-blind, placebo (PBO)-controlled, randomised withdrawal-retreatment period (RWRP). Patients who completed the originating 52-week COAST-V, COAST-W or COAST-X studies entered a 24-week lead-in period and continued either 80 mg IXE every 2 (Q2W) or 4 weeks (Q4W). Patients who achieved remission (an Ankylosing Spondylitis Disease Activity Score (ASDAS)<1.3 at least once at week 16 or week 20, and <2.1 at both visits) were randomly assigned equally at week 24 to continue IXE Q4W, IXE Q2W or withdraw to PBO in a blinded fashion. The primary endpoint was the proportion of flare-free patients (flare: ASDAS≥2.1 at two consecutive visits or ASDAS>3.5 at any visit) after the 40-week RWRP, with time-to-flare as a major secondary endpoint.
Results: Of 773 enrolled patients, 741 completed the 24-week lead-in period and 155 entered the RWRP. Forty weeks after randomised withdrawal, 83.3% of patients in the combined IXE (85/102, p<0.001), IXE Q4W (40/48, p=0.003) and IXE Q2W (45/54, p=0.001) groups remained flare-free versus 54.7% in the PBO group (29/53). Continuing IXE significantly delayed time-to-flare versus PBO, with most patients remaining flare-free for up to 20 weeks after IXE withdrawal.
Conclusions: Patients with axSpA who continued treatment with IXE were significantly less likely to flare and had significantly delayed time-to-flare compared with patients who withdrew to PBO.
Keywords: ankylosing; antirheumatic agents; biological therapy; immune system diseases; spondylitis.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RBML reports X-ray/MRI reading fees from Rheumatology Consultancy BV and personal fees from AbbVie, UCB, Pfizer, Eli Lilly and Company, Novartis and Celgene. LSG reports personal fees from AbbVie, Eli Lilly and Company, Gilead, Galapagos and GlaxoSmithKline; grants and personal fees from Novartis, Pfizer and UCB. DP reports honorarium from Eli Lilly and Company; grants and personal fees from AbbVie, Eli Lilly and Company, MSD, Novartis and Pfizer; and personal fees from Bristol-Myers Squibb, Roche, UCB, Biocad, GlaxoSmithKline and Gilead. PR reports personal fees from AbbVie, Amgen, BMS, Celgene, Eli Lilly and Company, Merck, Pfizer and UCB; and grants and personal fees from Janssen and Novartis. MH, XL, SLL, DA and HC report personal fees and stock ownership from Eli Lilly and Company. FVdB reports personal fees from AbbVie, Celgene, Eli Lilly and Company, Galapagos, Gilead, Janssen, Novartis, Pfizer and UCB.
Figures

References
-
- López-Medina C, Ramiro S, van der Heijde D, et al. . Characteristics and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open 2019;5:e001108. 10.1136/rmdopen-2019-001108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

