The Fetus with Ganglionic Eminence Abnormality: Head Size and Extracranial Sonographic Findings Predict Genetic Diagnoses and Postnatal Outcomes
- PMID: 33958329
- PMCID: PMC8367629
- DOI: 10.3174/ajnr.A7131
The Fetus with Ganglionic Eminence Abnormality: Head Size and Extracranial Sonographic Findings Predict Genetic Diagnoses and Postnatal Outcomes
Abstract
Background and purpose: Ganglionic eminence abnormalities on fetal MR imaging are associated with cerebral malformations. Their presumed genetic basis and associated postnatal outcomes remain largely unknown. We aimed to elucidate these through a multicenter study.
Materials and methods: Between January 2010 and June 2020, seven hospitals in 2 countries performing fetal MR imaging examinations identified fetal MR imaging studies demonstrating ganglionic eminence enlargement, cavitation, or both. Cases with no genetic diagnosis, no whole exome sequencing, or no outcome of a liveborn child were excluded. Head size was classified as large (fronto-occipital diameter > 95th centile), small (fronto-occipital diameter <5th centile), or normal.
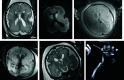
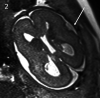
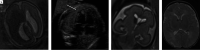
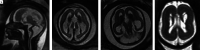
Results: Twenty-two fetuses with ganglionic eminence abnormalities were identified. Of 8 with large heads, 2 were diagnosed with MTOR mutations; 1 with PIK3CA mutation-producing megalencephaly, polymicrogyria, polydactyly, hydrocephalus (MPPH) syndrome; 3 with TSC mutations; 1 with megalencephaly capillary malformation syndrome; and 1 with hemimegalencephaly. Cardiac rhabdomyoma was present prenatally in all cases of TSC; mutation postaxial polydactyly accompanied megalencephaly capillary malformation and MPPH. Of 12 fetuses with small heads, 7 had TUBA1A mutations, 1 had a TUBB3 mutation, 2 had cobblestone lissencephaly postnatally with no genetic diagnosis, 1 had a PDHA1 mutation, and 1 had a fetal akinesia dyskinesia sequence with no pathogenic mutation on trio whole exome sequencing. One of the fetuses with a normal head size had an OPHN1 mutation with postnatal febrile seizures, and the other had peri-Sylvian polymicrogyria, seizures, and severe developmental delay but no explanatory mutation on whole exome sequencing.
Conclusions: Fetal head size and extracranial prenatal sonographic findings can refine the phenotype and facilitate genetic diagnosis when ganglionic eminence abnormality is diagnosed with MR imaging.
© 2021 by American Journal of Neuroradiology.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
