SPLUNC1: a novel marker of cystic fibrosis exacerbations
- PMID: 33958427
- PMCID: PMC8571118
- DOI: 10.1183/13993003.00507-2020
SPLUNC1: a novel marker of cystic fibrosis exacerbations
Abstract
Background: Acute pulmonary exacerbations (AE) are episodes of clinical worsening in cystic fibrosis (CF), often precipitated by infection. Timely detection is critical to minimise morbidity and lung function declines associated with acute inflammation during AE. Based on our previous observations that airway protein short palate lung nasal epithelium clone 1 (SPLUNC1) is regulated by inflammatory signals, we investigated the use of SPLUNC1 fluctuations to diagnose and predict AE in CF.
Methods: We enrolled CF participants from two independent cohorts to measure AE markers of inflammation in sputum and recorded clinical outcomes for a 1-year follow-up period.
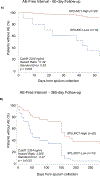
Results: SPLUNC1 levels were high in healthy controls (n=9, 10.7 μg·mL-1), and significantly decreased in CF participants without AE (n=30, 5.7 μg·mL-1; p=0.016). SPLUNC1 levels were 71.9% lower during AE (n=14, 1.6 μg·mL-1; p=0.0034) regardless of age, sex, CF-causing mutation or microbiology findings. Cytokines interleukin-1β and tumour necrosis factor-α were also increased in AE, whereas lung function did not decrease consistently. Stable CF participants with lower SPLUNC1 levels were much more likely to have an AE at 60 days (hazard ratio (HR)±se 11.49±0.83; p=0.0033). Low-SPLUNC1 stable participants remained at higher AE risk even 1 year after sputum collection (HR±se 3.21±0.47; p=0.0125). SPLUNC1 was downregulated by inflammatory cytokines and proteases increased in sputum during AE.
Conclusion: In acute CF care, low SPLUNC1 levels could support a decision to increase airway clearance or to initiate pharmacological interventions. In asymptomatic, stable patients, low SPLUNC1 levels could inform changes in clinical management to improve long-term disease control and clinical outcomes in CF.
Copyright ©The authors 2021. For reproduction rights and permissions contact permissions@ersnet.org.
Conflict of interest statement
Conflict of interest: S. Khanal has nothing to disclose. Conflict of interest: M. Webster has nothing to disclose. Conflict of interest: N. Niu has nothing to disclose. Conflict of interest: J. Zielonka has nothing to disclose. Conflict of interest: M. Nunez has nothing to disclose. Conflict of interest: G. Chupp reports other (advisor, clinical trial investigator, speaker) from Genentech, AstraZeneca and Sanofi-Regeneron, other (advisor, clinical trial investigator) from GSK, TEVA, Boehringer Ingelheim and Circassia, outside the submitted work. Conflict of interest: M.D. Slade has nothing to disclose. Conflict of interest: L. Cohn reports other (lectures) from Genentech, other (advisory board work) from Novartis, AstraZeneca, GlaxoSmithKline, Regeneron, Pieris and Sanofi, outside the submitted work. Conflict of interest: M. Sauler has nothing to disclose. Conflict of interest: J.L. Gomez has nothing to disclose. Conflict of interest: R. Tarran reports other (founder and equity) from Eldec Pharmaceuticals, outside the submitted work; and has a patent Peptide inhibitors of Ca2+ channels pending, a patent Peptide inhibitors of sodium channels with royalties paid, and a patent Regulation of sodium channels by PLUNC proteins with royalties paid. Conflict of interest: L. Sharma has nothing to disclose. Conflict of interest: C.S. Dela Cruz has nothing to disclose. Conflict of interest: M. Egan has nothing to disclose. Conflict of interest: T. Laguna reports grants from National Institutes of Health and Cystic Fibrosis Foundation, other (advisory board honorarium) from Vertex, outside the submitted work. Conflict of interest: C.J. Britto has nothing to disclose.
Figures





Comment in
-
SPLUNC1 is a novel marker of disease severity and airway infection in bronchiectasis.Eur Respir J. 2021 Nov 11;58(5):2101840. doi: 10.1183/13993003.01840-2021. Print 2021 Nov. Eur Respir J. 2021. PMID: 34413156 No abstract available.
-
SPLUNC1 comes of age? Predicting acute exacerbations in cystic fibrosis.Eur Respir J. 2021 Nov 11;58(5):2101569. doi: 10.1183/13993003.01569-2021. Print 2021 Nov. Eur Respir J. 2021. PMID: 34764214 No abstract available.
Similar articles
-
SPLUNC1 as a biomarker of pulmonary exacerbations in children with cystic fibrosis.J Cyst Fibros. 2024 Mar;23(2):288-292. doi: 10.1016/j.jcf.2024.02.009. Epub 2024 Feb 27. J Cyst Fibros. 2024. PMID: 38413298
-
SPX-101 is stable in and retains function after exposure to cystic fibrosis sputum.J Cyst Fibros. 2019 Mar;18(2):244-250. doi: 10.1016/j.jcf.2018.06.002. Epub 2018 Jun 20. J Cyst Fibros. 2019. PMID: 29936069
-
Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis.Respir Res. 2007 Nov 7;8(1):79. doi: 10.1186/1465-9921-8-79. Respir Res. 2007. PMID: 17988392 Free PMC article.
-
The Multifunctional Roles of Short Palate, Lung, and Nasal Epithelium Clone 1 in Regulating Airway Surface Liquid and Participating in Airway Host Defense.J Interferon Cytokine Res. 2021 Apr;41(4):139-148. doi: 10.1089/jir.2020.0141. J Interferon Cytokine Res. 2021. PMID: 33885339 Review.
-
Differential short palate, lung, and nasal epithelial clone 1 suppression in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps: implications for pathogenesis and treatment.Curr Opin Allergy Clin Immunol. 2016 Feb;16(1):31-8. doi: 10.1097/ACI.0000000000000228. Curr Opin Allergy Clin Immunol. 2016. PMID: 26658012 Review.
Cited by
-
Lung antimicrobial proteins and peptides: from host defense to therapeutic strategies.Physiol Rev. 2024 Oct 1;104(4):1643-1677. doi: 10.1152/physrev.00039.2023. Epub 2024 Jul 25. Physiol Rev. 2024. PMID: 39052018 Review.
-
Evaluation of serum VIP and aCGRP during pulmonary exacerbation in cystic fibrosis: A longitudinal pilot study of patients undergoing antibiotic therapy.PLoS One. 2023 May 5;18(5):e0284511. doi: 10.1371/journal.pone.0284511. eCollection 2023. PLoS One. 2023. PMID: 37146001 Free PMC article.
-
Specific Inhibition of Orai1-mediated Calcium Signalling Resolves Inflammation and Clears Bacteria in an Acute Respiratory Distress Syndrome Model.Am J Respir Crit Care Med. 2024 Mar 15;209(6):703-715. doi: 10.1164/rccm.202308-1393OC. Am J Respir Crit Care Med. 2024. PMID: 37972349 Free PMC article.
-
PLUNC downregulates the expression of PD-L1 by inhibiting the interaction of DDX17/β-catenin in nasopharyngeal carcinoma.J Pathol Transl Med. 2025 Jan;59(1):68-83. doi: 10.4132/jptm.2024.11.27. Epub 2025 Jan 15. J Pathol Transl Med. 2025. PMID: 39815745 Free PMC article.
-
A year in review: Real world evidence, functional monitoring and emerging therapeutics in 2021.J Cyst Fibros. 2022 Mar;21(2):191-196. doi: 10.1016/j.jcf.2022.02.014. Epub 2022 Mar 7. J Cyst Fibros. 2022. PMID: 35272931 Free PMC article. Review. No abstract available.
References
-
- Foundation, C. F. 2018 Annual Data Report. (Cystic Fibrosis Foundation, Bethesda, Maryland, 2019).
-
- Welsh MJ RB, Accurso F, Cutting GR. in The metabolic and molecular basis of inherited disease (ed Beaudet AL Scriver CR, Sly WS,Valle D,Childs B,Vogelstein B) 5121–5189 (McGraw-Hill, 2001).
-
- Foundation, C. F. 2019 Annual Data Report. (Cystic Fibrosis Foundation, Bethesda, Maryland, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
