Intrasubunit and Intersubunit Steroid Binding Sites Independently and Additively Mediate α 1 β 2 γ 2L GABAA Receptor Potentiation by the Endogenous Neurosteroid Allopregnanolone
- PMID: 33958479
- PMCID: PMC8256884
- DOI: 10.1124/molpharm.121.000268
Intrasubunit and Intersubunit Steroid Binding Sites Independently and Additively Mediate α 1 β 2 γ 2L GABAA Receptor Potentiation by the Endogenous Neurosteroid Allopregnanolone
Abstract
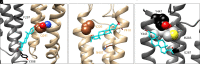
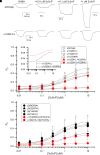
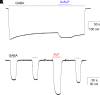
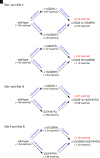
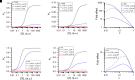
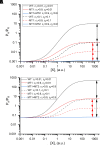
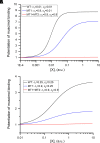
Prior work employing functional analysis, photolabeling, and X-ray crystallography have identified three distinct binding sites for potentiating steroids in the heteromeric GABAA receptor. The sites are located in the membrane-spanning domains of the receptor at the β-α subunit interface (site I) and within the α (site II) and β subunits (site III). Here, we have investigated the effects of mutations to these sites on potentiation of the rat α1β2γ2L GABAA receptor by the endogenous neurosteroid allopregnanolone (3α5αP). The mutations were introduced alone or in combination to probe the additivity of effects. We show that the effects of amino acid substitutions in sites I and II are energetically additive, indicating independence of the actions of the two steroid binding sites. In site III, none of the mutations tested reduced potentiation by 3α5αP, nor did a mutation in site III modify the effects of mutations in sites I or II. We infer that the binding sites for 3α5αP act independently. The independence of steroid action at each site is supported by photolabeling data showing that mutations in either site I or site II selectively change steroid orientation in the mutated site without affecting labeling at the unmutated site. The findings are discussed in the context of linking energetic additivity to empirical changes in receptor function and ligand binding. SIGNIFICANCE STATEMENT: Prior work has identified three distinct binding sites for potentiating steroids in the heteromeric γ-aminobutyric acid type A receptor. This study shows that the sites act independently and additively in the presence of the steroid allopregnanolone and provide estimates of energetic contributions made by steroid binding to each site.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
Photoaffinity labeling identifies an intersubunit steroid-binding site in heteromeric GABA type A (GABAA) receptors.J Biol Chem. 2020 Aug 14;295(33):11495-11512. doi: 10.1074/jbc.RA120.013452. Epub 2020 Jun 15. J Biol Chem. 2020. PMID: 32540960 Free PMC article.
-
A neurosteroid potentiation site can be moved among GABAA receptor subunits.J Physiol. 2012 Nov 15;590(22):5739-47. doi: 10.1113/jphysiol.2012.237255. Epub 2012 Sep 17. J Physiol. 2012. PMID: 22988137 Free PMC article.
-
Ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the alpha1beta2gamma2L GABAA receptor.Mol Pharmacol. 2007 Feb;71(2):461-72. doi: 10.1124/mol.106.029942. Epub 2006 Nov 14. Mol Pharmacol. 2007. PMID: 17105870
-
Neurosteroid Binding and Actions on GABAA Receptors.Juntendo Iji Zasshi. 2024 May 24;70(3):239-244. doi: 10.14789/jmj.JMJ24-0002-R. eCollection 2024. Juntendo Iji Zasshi. 2024. PMID: 39429691 Free PMC article. Review.
-
GABAA receptor modulating steroid antagonists (GAMSA) are functional in vivo.J Steroid Biochem Mol Biol. 2016 Jun;160:98-105. doi: 10.1016/j.jsbmb.2015.10.019. Epub 2015 Oct 30. J Steroid Biochem Mol Biol. 2016. PMID: 26523675 Review.
Cited by
-
Synthesis and evaluation of photoaffinity labeling reagents for identifying binding sites of sulfated neurosteroids on NMDA and GABAA receptors.RSC Adv. 2024 Nov 13;14(49):36352-36369. doi: 10.1039/d4ra07074g. eCollection 2024 Nov 11. RSC Adv. 2024. PMID: 39539530 Free PMC article.
-
Neurosteroids and their potential as a safer class of general anesthetics.J Anesth. 2024 Apr;38(2):261-274. doi: 10.1007/s00540-023-03291-4. Epub 2024 Jan 22. J Anesth. 2024. PMID: 38252143 Free PMC article. Review.
-
A propofol binding site in the voltage sensor domain mediates inhibition of HCN1 channel activity.Sci Adv. 2025 Jan 3;11(1):eadr7427. doi: 10.1126/sciadv.adr7427. Epub 2025 Jan 3. Sci Adv. 2025. PMID: 39752505 Free PMC article.
-
(+)-Catharanthine potentiates the GABAA receptor by binding to a transmembrane site at the β(+)/α(-) interface near the TM2-TM3 loop.Biochem Pharmacol. 2022 May;199:114993. doi: 10.1016/j.bcp.2022.114993. Epub 2022 Mar 15. Biochem Pharmacol. 2022. PMID: 35304861 Free PMC article.
-
Activation of the Rat α1β2ε GABAA Receptor by Orthosteric and Allosteric Agonists.Biomolecules. 2022 Jun 21;12(7):868. doi: 10.3390/biom12070868. Biomolecules. 2022. PMID: 35883422 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

