Differential In Vitro Pharmacological Profiles of Structurally Diverse Nociceptin Receptor Agonists in Activating G Protein and Beta-Arrestin Signaling at the Human Nociceptin Opioid Receptor
- PMID: 33958480
- PMCID: PMC8256882
- DOI: 10.1124/molpharm.120.000076
Differential In Vitro Pharmacological Profiles of Structurally Diverse Nociceptin Receptor Agonists in Activating G Protein and Beta-Arrestin Signaling at the Human Nociceptin Opioid Receptor
Abstract
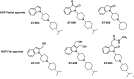
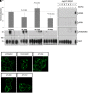
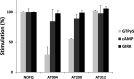
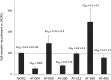
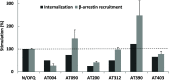
Agonists at the nociceptin opioid peptide receptor (NOP) are under investigation as therapeutics for nonaddicting analgesia, opioid use disorder, Parkinson's disease, and other indications. NOP full and partial agonists have both been of interest, particularly since NOP partial agonists show a reduced propensity for behavioral disruption than NOP full agonists. Here, we investigated the in vitro pharmacological properties of chemically diverse NOP receptor agonists in assays measuring functional activation of the NOP receptor such as guanosine 5'-O-[gamma-thio]triphosphate (GTPγS) binding, cAMP inhibition, G protein-coupled inwardly rectifying potassium (GIRK) channel activation, phosphorylation, β-arrestin recruitment and receptor internalization. When normalized to the efficacy of the natural agonist nociceptin/orphanin FQ (N/OFQ), we found that different functional assays that measure intrinsic activity produce inconsistent levels of agonist efficacy, particularly for ligands that were partial agonists. Agonist efficacy obtained in the GTPγS assay tended to be lower than that in the cAMP and GIRK assays. These structurally diverse NOP agonists also showed differential receptor phosphorylation profiles at the phosphosites we examined and induced varying levels of receptor internalization. Interestingly, although the rank order for β-arrestin recruitment by these NOP agonists was consistent with their ability to induce receptor internalization, their phosphorylation signatures at the time point we investigated were not indicative of the levels of β-arrestin recruitment or internalization induced by these agonists. It is possible that other phosphorylation sites, yet to be identified, drive the recruitment of NOP receptor ensembles and subsequent receptor trafficking by some nonpeptide NOP agonists. These findings potentially help understand NOP agonist pharmacology in the context of ligand-activated receptor trafficking. SIGNIFICANCE STATEMENT: Chemically diverse agonist ligands at the nociceptin opioid receptor G protein-coupled receptor showed differential efficacy for activating downstream events after receptor binding, in a suite of functional assays measuring guanosine 5'-O-[gamma-thio]triphosphate binding, cAMP inhibition, G protein-coupled inwardly rectifying protein channel activation, β-arrestin recruitment, receptor internalization and receptor phosphorylation. These analyses provide a context for understanding nociceptin opioid peptide receptor (NOP) agonist pharmacology driven by ligand-induced differential NOP receptor signaling.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
Pharmacological Profile of Nociceptin/Orphanin FQ Receptors Interacting with G-Proteins and β-Arrestins 2.PLoS One. 2015 Aug 6;10(8):e0132865. doi: 10.1371/journal.pone.0132865. eCollection 2015. PLoS One. 2015. PMID: 26248189 Free PMC article.
-
Beta-arrestin 2 rather than G protein efficacy determines the anxiolytic-versus antidepressant-like effects of nociceptin/orphanin FQ receptor ligands.Neuropharmacology. 2016 Jun;105:434-442. doi: 10.1016/j.neuropharm.2016.02.003. Epub 2016 Feb 8. Neuropharmacology. 2016. PMID: 26867504 Free PMC article.
-
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.Pharmacol Ther. 2014 Mar;141(3):283-99. doi: 10.1016/j.pharmthera.2013.10.011. Epub 2013 Nov 1. Pharmacol Ther. 2014. PMID: 24189487 Free PMC article. Review.
-
Agonist-selective NOP receptor phosphorylation correlates in vitro and in vivo and reveals differential post-activation signaling by chemically diverse agonists.Sci Signal. 2019 Mar 26;12(574):eaau8072. doi: 10.1126/scisignal.aau8072. Sci Signal. 2019. PMID: 30914485 Free PMC article.
-
Pharmacological characterization of nociceptin/orphanin FQ receptors, a novel opioid receptor family, in the midbrain periaqueductal gray.Ann N Y Acad Sci. 2004 Oct;1025:398-403. doi: 10.1196/annals.1316.049. Ann N Y Acad Sci. 2004. PMID: 15542742 Review.
Cited by
-
Cannabinoids: Potential for Modulation and Enhancement When Combined with Vitamin B12 in Case of Neurodegenerative Disorders.Pharmaceuticals (Basel). 2024 Jun 20;17(6):813. doi: 10.3390/ph17060813. Pharmaceuticals (Basel). 2024. PMID: 38931480 Free PMC article. Review.
References
-
- Adapa ID, Toll L (1997) Relationship between binding affinity and functional activity of nociceptin/orphanin FQ. Neuropeptides 31:403–408. - PubMed
-
- Adham N, Ellerbrock B, Hartig P, Weinshank RL, Branchek T (1993) Receptor reserve masks partial agonist activity of drugs in a cloned rat 5-hydroxytryptamine1B receptor expression system. Mol Pharmacol 43:427–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

