Progesterone promotes immunomodulation and tumor development in the murine mammary gland
- PMID: 33958486
- PMCID: PMC8103939
- DOI: 10.1136/jitc-2020-001710
Progesterone promotes immunomodulation and tumor development in the murine mammary gland
Abstract
Background: Clinical studies have linked usage of progestins (synthetic progesterone [P4]) to breast cancer risk. However, little is understood regarding the role of native P4, signaling through the progesterone receptor (PR), in breast tumor formation. Recently, we reported a link between PR and immune signaling pathways, showing that P4/PR can repress type I interferon signaling pathways. Given these findings, we sought to investigate whether P4/PR drive immunomodulation in the mammary gland and promote tumor formation.
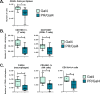
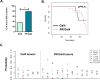
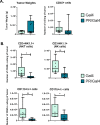
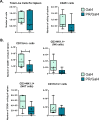
Methods: To determine the effect of P4 on immune cell populations in the murine mammary gland, mice were treated with P4 or placebo pellets for 21 days. Immune cell populations in the mammary gland, spleen, and inguinal lymph nodes were subsequently analyzed by flow cytometry. To assess the effect of PR overexpression on mammary gland tumor development as well as immune cell populations in the mammary gland, a transgenic mouse model was used in which PR was overexpressed throughout the entire mouse. Immune cell populations were assessed in the mammary glands, spleens, and inguinal lymph nodes of 6-month-old transgenic and control mice by flow cytometry. Transgenic mice were also monitored for mammary gland tumor development over a 2-year time span. Following development of mammary gland tumors, immune cell populations in the tumors and spleens of transgenic and control mice were analyzed by flow cytometry.
Results: We found that mice treated with P4 exhibited changes in the mammary gland indicative of an inhibited immune response compared with placebo-treated mice. Furthermore, transgenic mice with PR overexpression demonstrated decreased numbers of immune cell populations in their mammary glands, lymph nodes, and spleens. On long-term monitoring, we determined that multiparous PR-overexpressing mice developed significantly more mammary gland tumors than control mice. Additionally, tumors from PR-overexpressing mice contained fewer infiltrating immune cells. Finally, RNA sequencing analysis of tumor samples revealed that immune-related gene signatures were lower in tumors from PR-overexpressing mice as compared with control mice.
Conclusion: Together, these findings offer a novel mechanism of P4-driven mammary gland tumor development and provide rationale in investigating the usage of antiprogestin therapies to promote immune-mediated elimination of mammary gland tumors.
Keywords: adaptive immunity; breast neoplasms; immunomodulation; lymphocytes; tumor microenvironment; tumor-infiltrating.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CAL is a scientific advisor for Context Therapeutics, Inc, outside the submitted work; MAM is a consultant for Johnson & Johnson Global Services, outside the submitted work; JMB reports grants from Genentech/Roche, Bristol Myers Squibb, and Incyte Corporation, and consulting/expert witness fees from Novartis, outside the submitted work. In addition, JMB has patents and patents pending on predictive factors for immunotherapy and chemotherapy outcome in cancer, outside the submitted work. MLA is listed as a coinventor on a provisional patent application on methods to predict therapeutic outcome using blood-based gene expression patterns, that is owned by Vanderbilt University Medical Center, and is currently unlicensed; outside the submitted work. No other authors have any disclosures.
Figures


Similar articles
-
Progesterone receptor-dependent downregulation of MHC class I promotes tumor immune evasion and growth in breast cancer.J Immunother Cancer. 2025 Mar 18;13(3):e010179. doi: 10.1136/jitc-2024-010179. J Immunother Cancer. 2025. PMID: 40102028 Free PMC article.
-
BRCA1-associated mammary tumorigenesis is dependent on estrogen rather than progesterone signaling.J Pathol. 2018 Sep;246(1):41-53. doi: 10.1002/path.5105. Epub 2018 Jul 4. J Pathol. 2018. PMID: 29877575
-
The hyperplastic phenotype in PR-A and PR-B transgenic mice: lessons on the role of estrogen and progesterone receptors in the mouse mammary gland and breast cancer.Vitam Horm. 2013;93:185-201. doi: 10.1016/B978-0-12-416673-8.00012-5. Vitam Horm. 2013. PMID: 23810007 Review.
-
Distinct temporal and spatial activities of RU486 on progesterone receptor function in reproductive organs of ovariectomized mice.Endocrinology. 2007 May;148(5):2471-86. doi: 10.1210/en.2006-1561. Epub 2007 Feb 15. Endocrinology. 2007. PMID: 17303655
-
The biology of progesterone receptor in the normal mammary gland and in breast cancer.Mol Cell Endocrinol. 2012 Jun 24;357(1-2):4-17. doi: 10.1016/j.mce.2011.10.030. Epub 2011 Dec 13. Mol Cell Endocrinol. 2012. PMID: 22193050 Free PMC article. Review.
Cited by
-
Senescence-associated secretory phenotype (SASP) and uterine fibroids: Association with PD-L1 activation and collagen deposition.Ageing Res Rev. 2024 Jun;97:102314. doi: 10.1016/j.arr.2024.102314. Epub 2024 Apr 24. Ageing Res Rev. 2024. PMID: 38670462 Free PMC article. Review.
-
Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac Progenitor Specification during Mouse Gastrulation.Int J Mol Sci. 2022 Sep 7;23(18):10307. doi: 10.3390/ijms231810307. Int J Mol Sci. 2022. PMID: 36142249 Free PMC article.
-
Progesterone receptor-dependent downregulation of MHC class I promotes tumor immune evasion and growth in breast cancer.J Immunother Cancer. 2025 Mar 18;13(3):e010179. doi: 10.1136/jitc-2024-010179. J Immunother Cancer. 2025. PMID: 40102028 Free PMC article.
-
Role of gonadally synthesized steroid hormones in the colorectal cancer microenvironment.Front Oncol. 2023 Dec 5;13:1323826. doi: 10.3389/fonc.2023.1323826. eCollection 2023. Front Oncol. 2023. PMID: 38115900 Free PMC article. Review.
-
Spatial profiling reveals unique immune microenvironment in premenopausal triple-negative breast cancer associated with therapy response.J Transl Med. 2025 Jul 10;23(1):761. doi: 10.1186/s12967-025-06786-8. J Transl Med. 2025. PMID: 40640880 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
