Diversification, selective sweep, and body size in the invasive Palearctic alfalfa weevil infected with Wolbachia
- PMID: 33958611
- PMCID: PMC8102540
- DOI: 10.1038/s41598-021-88770-y
Diversification, selective sweep, and body size in the invasive Palearctic alfalfa weevil infected with Wolbachia
Abstract
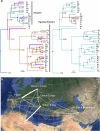
The alfalfa weevil Hypera postica, native to the Western Palearctic, is an invasive legume pest with two divergent mitochondrial clades in its invading regions, the Western clade and the Eastern/Egyptian clade. However, knowledge regarding the native populations is limited. The Western clade is infected with the endosymbiotic bacteria Wolbachia that cause cytoplasmic incompatibility in host weevils. Our aim was to elucidate the spatial genetic structure of this insect and the effect of Wolbachia on its population diversity. We analyzed two mitochondrial and two nuclear genes of the weevil from its native ranges. The Western clade was distributed in western/central Europe, whereas the Eastern/Egyptian clade was distributed from the Mediterranean basin to central Asia. Intermediate mitotypes were found from the Balkans to central Asia. Most Western clade individuals in western Europe were infected with an identical Wolbachia strain. Mitochondrial genetic diversity of the infected individuals was minimal. The infected clades demonstrated a higher nonsynonymous/synonymous substitution rate ratio than the uninfected clades, suggesting a higher fixation of nonsynonymous mutations due to a selective sweep by Wolbachia. Trans-Mediterranean and within-European dispersal routes were supported. We suggest that the ancestral populations diversified by geographic isolation due to glaciations and that the diversity was reduced in the west by a recent Wolbachia-driven sweep(s). The intermediate clade exhibited a body size and host plant that differed from the other clades. Pros and cons of the possible use of infected-clade males to control uninfected populations are discussed.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Host-plant dependent population genetics of the invading weevil Hypera postica.Bull Entomol Res. 2015 Feb;105(1):92-100. doi: 10.1017/S0007485314000728. Epub 2014 Oct 22. Bull Entomol Res. 2015. PMID: 25336385
-
Global genetic diversity, lineage distribution, and Wolbachia infection of the alfalfa weevil Hypera postica (Coleoptera: Curculionidae).Ecol Evol. 2019 Aug 6;9(17):9546-9563. doi: 10.1002/ece3.5474. eCollection 2019 Sep. Ecol Evol. 2019. PMID: 31534674 Free PMC article.
-
Selective sweep of Wolbachia and parthenogenetic host genomes - the example of the weevil Eusomus ovulum.Insect Mol Biol. 2016 Dec;25(6):701-711. doi: 10.1111/imb.12255. Epub 2016 Jul 20. Insect Mol Biol. 2016. PMID: 27438898
-
Wolbachia-driven selective sweep in a range expanding insect species.BMC Ecol Evol. 2021 Sep 25;21(1):181. doi: 10.1186/s12862-021-01906-6. BMC Ecol Evol. 2021. PMID: 34563127 Free PMC article.
-
Assessment of the global pattern of genetic diversity in Echinococcus multilocularis inferred by mitochondrial DNA sequences.Vet Parasitol. 2018 Oct 15;262:30-41. doi: 10.1016/j.vetpar.2018.09.013. Epub 2018 Sep 27. Vet Parasitol. 2018. PMID: 30389009 Review.
Cited by
-
Validating a Mitochondrial Sweep Accompanying the Rapid Spread of a Maternally Inherited Symbiont.Methods Mol Biol. 2024;2739:239-247. doi: 10.1007/978-1-0716-3553-7_15. Methods Mol Biol. 2024. PMID: 38006556
-
Wolbachia infection facilitates adaptive increase in male egg size in response to environmental changes.Sci Rep. 2025 Apr 16;15(1):13213. doi: 10.1038/s41598-025-96680-6. Sci Rep. 2025. PMID: 40240454 Free PMC article.
References
-
- Karsten M, van Vuuren BJ, Addison P, Terblanche JS. Deconstructing intercontinental invasion pathway hypotheses of the Mediterranean fruit fly (Ceratitis capitata) using a Bayesian inference approach: Are port interceptions and quarantine protocols successfully preventing new invasions? Divers. Distrib. 2015;21:813–825. doi: 10.1111/ddi.12333. - DOI
-
- Kébé K, et al. Global phylogeography of the insect pest Callosobruchus maculatus (Coleoptera: Bruchinae) relates to the history of its main host Vigna unguiculata. J. Biogeogr. 2017;44:2515–2526. doi: 10.1111/jbi.13052. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

