Steroid hormone bioavailability is controlled by the lymphatic system
- PMID: 33958648
- PMCID: PMC8102502
- DOI: 10.1038/s41598-021-88508-w
Steroid hormone bioavailability is controlled by the lymphatic system
Abstract
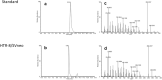
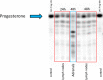
The steroid hormone progesterone accounts for immune tolerance in pregnancy. Enhanced progesterone metabolism to 6α-OH-pregnanolone occurs in complicated pregnancies such as in preeclampsia with preterm delivery or intrauterine growth restriction, and in cancer. As lymphatic endothelial cells (LECs) promote tumor immunity, we hypothesized that human LECs modify progesterone bioavailability. Primary human LECs and mice lymph nodes were incubated with progesterone and progesterone metabolism was analyzed by thin layer chromatography and liquid chromatography-mass spectrometry. Expression of steroidogenic enzymes, down-stream signal and steroid hormone receptors was assessed by Real-time PCR. The placental cell line HTR-8/SV neo was used as reference. The impact of the progesterone metabolites of interest was investigated on the immune system by fluorescence-activated cell sorting analysis. LECs metabolize progesterone to 6α-OH-pregnanolone and reactivate progesterone from a precursor. LECs highly express 17β-hydroxysteroid dehydrogenase 2 and are therefore antiandrogenic and antiestrogenic. LECs express several steroid hormone receptors and PIBF1. Progesterone and its metabolites reduced TNF-α and IFN-γ production in CD4+ and CD8+ T cells. LECs modify progesterone bioavailability and are a target of steroid hormones. Given the global area represented by LECs, they might have a critical immunomodulatory control in pregnancy and cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Interferon-γ and tumor necrosis factor-α promote the ability of human placenta-derived mesenchymal stromal cells to express programmed death ligand-2 and induce the differentiation of CD4(+)interleukin-10(+) and CD8(+)interleukin-10(+)Treg subsets.Cytotherapy. 2015 Nov;17(11):1560-71. doi: 10.1016/j.jcyt.2015.07.018. Cytotherapy. 2015. PMID: 26432559
-
Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells.Blood. 2012 Dec 6;120(24):4772-82. doi: 10.1182/blood-2012-04-427013. Epub 2012 Sep 19. Blood. 2012. PMID: 22993390 Free PMC article.
-
Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice.Arthritis Res Ther. 2016 Mar 12;18:62. doi: 10.1186/s13075-016-0963-8. Arthritis Res Ther. 2016. PMID: 26970913 Free PMC article.
-
Roles of cytokines and progesterone in the regulation of the nitric oxide generating system in bovine luteal endothelial cells.Mol Reprod Dev. 2012 Oct;79(10):689-96. doi: 10.1002/mrd.22075. Epub 2012 Sep 11. Mol Reprod Dev. 2012. PMID: 22847916
-
Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors.Prog Neurobiol. 2014 Feb;113:6-39. doi: 10.1016/j.pneurobio.2013.09.004. Epub 2013 Oct 27. Prog Neurobiol. 2014. PMID: 24172649 Review.
Cited by
-
Early Pregnancy Regulates Expression of IkappaB Family in Ovine Spleen and Lymph Nodes.Int J Mol Sci. 2023 Mar 8;24(6):5156. doi: 10.3390/ijms24065156. Int J Mol Sci. 2023. PMID: 36982231 Free PMC article.
-
Regulatory T cells are paramount effectors in progesterone regulation of embryo implantation and fetal growth.JCI Insight. 2023 Jun 8;8(11):e162995. doi: 10.1172/jci.insight.162995. JCI Insight. 2023. PMID: 37191999 Free PMC article.
-
No extra-adrenal aldosterone production in various human cell lines.J Mol Endocrinol. 2024 Feb 1;72(3):e230100. doi: 10.1530/JME-23-0100. Print 2024 Apr 1. J Mol Endocrinol. 2024. PMID: 38175924 Free PMC article.
-
Allopregnanolone: Metabolism, Mechanisms of Action, and Its Role in Cancer.Int J Mol Sci. 2022 Dec 29;24(1):560. doi: 10.3390/ijms24010560. Int J Mol Sci. 2022. PMID: 36614002 Free PMC article. Review.
-
The lymphatic vascular system: much more than just a sewer.Cell Biosci. 2022 Sep 15;12(1):157. doi: 10.1186/s13578-022-00898-0. Cell Biosci. 2022. PMID: 36109802 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

