Repetitive mild head trauma induces activity mediated lifelong brain deficits in a novel Drosophila model
- PMID: 33958652
- PMCID: PMC8102574
- DOI: 10.1038/s41598-021-89121-7
Repetitive mild head trauma induces activity mediated lifelong brain deficits in a novel Drosophila model
Abstract
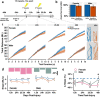
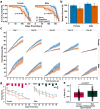
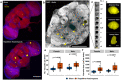
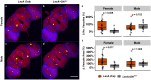
Mild head trauma, including concussion, can lead to chronic brain dysfunction and degeneration but the underlying mechanisms remain poorly understood. Here, we developed a novel head impact system to investigate the long-term effects of mild head trauma on brain structure and function, as well as the underlying mechanisms in Drosophila melanogaster. We find that Drosophila subjected to repetitive head impacts develop long-term deficits, including impaired startle-induced climbing, progressive brain degeneration, and shortened lifespan, all of which are substantially exacerbated in female flies. Interestingly, head impacts elicit an elevation in neuronal activity and its acute suppression abrogates the detrimental effects in female flies. Together, our findings validate Drosophila as a suitable model system for investigating the long-term effects of mild head trauma, suggest an increased vulnerability to brain injury in female flies, and indicate that early altered neuronal excitability may be a key mechanism linking mild brain trauma to chronic degeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Little DM, Geary EK, Moynihan M, Alexander A, Pennington M, Glang P, Schulze ET, Dretsch M, Pacifico A, Davis ML, Stevens AB, Huang JH. Imaging chronic traumatic brain injury as a risk factor for neurodegeneration. Alzheimers Dement. 2014;10(3 Suppl):S188–S195. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

