Novel capsid binder and PI4KIIIbeta inhibitors for EV-A71 replication inhibition
- PMID: 33958691
- PMCID: PMC8102518
- DOI: 10.1038/s41598-021-89271-8
Novel capsid binder and PI4KIIIbeta inhibitors for EV-A71 replication inhibition
Abstract
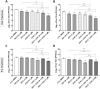
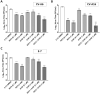
The Hand, Foot and Mouth Disease (HFMD) is a highly contagious viral illness generally manifests as a mild disease in young children and immunocompromised adults. It has however emerged as a significant public health threat in recent years as outbreaks have been occurring regularly, especially in the Asia-Pacific. The disease can result from infections by a wide variety of human enteroviruses, particularly, Enterovirus A71 (EV-A71) has garnered more attention due to its association with severe disease in infected patients. Despite the potential to result severe neurological complications or even fatality, there is currently no effective antiviral for treatment of EV-A71 infections and the only vaccines available are restricted to distribution in China. In this study, we report the in vitro and in vivo evaluation of two candidate antiviral compounds active against EV-A71, a viral capsid inhibitor (G197) and a novel host-targeting phosphatidylinositol 4-kinase III beta inhibitor (N373) which, especially when used in combination, can significantly improve the survival and pathology of infected mice.
Conflict of interest statement
All PI4KIIIβ analogues are covered in patent/patent applications owned by Curovir AB (JW and GA). YWT, WKY, RJWK, JJHC declare no potential conflict of interest.
Figures




Similar articles
-
Vascular endothelial growth factor receptor 2 as a potential host target for the inhibition of enterovirus replication.J Virol. 2024 Oct 22;98(10):e0112924. doi: 10.1128/jvi.01129-24. Epub 2024 Sep 17. J Virol. 2024. PMID: 39287389 Free PMC article.
-
Tanomastat exerts multi-targeted inhibitory effects on viral capsid dissociation and RNA replication in human enteroviruses.EBioMedicine. 2024 Sep;107:105277. doi: 10.1016/j.ebiom.2024.105277. Epub 2024 Sep 2. EBioMedicine. 2024. PMID: 39226680 Free PMC article.
-
Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71).Viruses. 2020 Feb 6;12(2):184. doi: 10.3390/v12020184. Viruses. 2020. PMID: 32041232 Free PMC article. Review.
-
Antiviral Peptides Targeting the Helicase Activity of Enterovirus Nonstructural Protein 2C.J Virol. 2021 May 24;95(12):e02324-20. doi: 10.1128/JVI.02324-20. Print 2021 May 24. J Virol. 2021. PMID: 33789997 Free PMC article.
-
Recent Advances in Enterovirus A71 Infection and Antiviral Agents.Lab Invest. 2024 Feb;104(2):100298. doi: 10.1016/j.labinv.2023.100298. Epub 2023 Nov 25. Lab Invest. 2024. PMID: 38008182 Review.
Cited by
-
EV-A71 Mechanism of Entry: Receptors/Co-Receptors, Related Pathways and Inhibitors.Viruses. 2023 Mar 18;15(3):785. doi: 10.3390/v15030785. Viruses. 2023. PMID: 36992493 Free PMC article. Review.
-
Targeting the Virus Capsid as a Tool to Fight RNA Viruses.Viruses. 2022 Jan 18;14(2):174. doi: 10.3390/v14020174. Viruses. 2022. PMID: 35215767 Free PMC article. Review.
-
Inhibition of EV71 replication by an interferon-stimulated gene product L3HYPDH.Virus Res. 2024 Apr;342:199336. doi: 10.1016/j.virusres.2024.199336. Epub 2024 Feb 13. Virus Res. 2024. PMID: 38342315 Free PMC article.
-
From the "One-Molecule, One-Target, One-Disease" Concept towards Looking for Multi-Target Therapeutics for Treating Non-Polio Enterovirus (NPEV) Infections.Pharmaceuticals (Basel). 2024 Sep 16;17(9):1218. doi: 10.3390/ph17091218. Pharmaceuticals (Basel). 2024. PMID: 39338380 Free PMC article. Review.
References
-
- Park SH, et al. Detection and characterization of enterovirus associated with herpangina and hand, foot, and mouth disease in Seoul, Korea. Clin. Lab. 2011;57(11–12):959–967. - PubMed
-
- Chang CS, et al. Design, synthesis, and antipicornavirus activity of 1-[5-(4-arylphenoxy)alkyl]-3-pyridin-4-ylimidazolidin-2-one derivatives. J. Med. Chem. 2005;48(10):3522–3535. - PubMed
-
- Chen TC, et al. Antiviral activity of pyridyl imidazolidinones against enterovirus 71 variants. J. Biomed. Sci. 2008;15(3):291–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

