A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase
- PMID: 33958765
- PMCID: PMC9793891
- DOI: 10.1038/s41564-021-00899-8
A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase
Abstract
The stalling global progress in the fight against malaria prompts the urgent need to develop new intervention strategies. Whilst engineered symbiotic bacteria have been shown to confer mosquito resistance to parasite infection, a major challenge for field implementation is to address regulatory concerns. Here, we report the identification of a Plasmodium-blocking symbiotic bacterium, Serratia ureilytica Su_YN1, isolated from the midgut of wild Anopheles sinensis in China that inhibits malaria parasites via secretion of an antimalarial lipase. Analysis of Plasmodium vivax epidemic data indicates that local malaria cases in Tengchong (Yunnan province, China) are significantly lower than imported cases and importantly, that the local vector A. sinensis is more resistant to infection by P. vivax than A. sinensis from other regions. Analysis of the gut symbiotic bacteria of mosquitoes from Yunnan province led to the identification of S. ureilytica Su_YN1. This bacterium renders mosquitoes resistant to infection by the human parasite Plasmodium falciparum or the rodent parasite Plasmodium berghei via secretion of a lipase that selectively kills parasites at various stages. Importantly, Su_YN1 rapidly disseminates through mosquito populations by vertical and horizontal transmission, providing a potential tool for blocking malaria transmission in the field.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
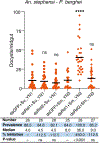
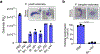

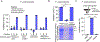


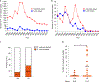


Similar articles
-
Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria.Science. 2017 Sep 29;357(6358):1399-1402. doi: 10.1126/science.aan5478. Epub 2017 Sep 28. Science. 2017. PMID: 28963255 Free PMC article.
-
Anti-mosquito midgut antibodies block development of Plasmodium falciparum and Plasmodium vivax in multiple species of Anopheles mosquitoes and reduce vector fecundity and survivorship.Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5228-33. doi: 10.1073/pnas.091447398. Epub 2001 Apr 17. Proc Natl Acad Sci U S A. 2001. PMID: 11309510 Free PMC article.
-
Optimization of a Membrane Feeding Assay for Plasmodium vivax Infection in Anopheles albimanus.PLoS Negl Trop Dis. 2016 Jun 29;10(6):e0004807. doi: 10.1371/journal.pntd.0004807. eCollection 2016 Jun. PLoS Negl Trop Dis. 2016. PMID: 27355210 Free PMC article.
-
The tripartite interactions between the mosquito, its microbiota and Plasmodium.Parasit Vectors. 2018 Mar 20;11(1):200. doi: 10.1186/s13071-018-2784-x. Parasit Vectors. 2018. PMID: 29558973 Free PMC article. Review.
-
Anopheles stephensi (Asian Malaria Mosquito).Trends Parasitol. 2021 Jun;37(6):571-572. doi: 10.1016/j.pt.2021.03.009. Epub 2021 Apr 14. Trends Parasitol. 2021. PMID: 33865712 Review. No abstract available.
Cited by
-
Symbiotic Bacteria System of Locusta migratoria Showed Antifungal Capabilities against Beauveria bassiana.Int J Mol Sci. 2023 Feb 5;24(4):3138. doi: 10.3390/ijms24043138. Int J Mol Sci. 2023. PMID: 36834550 Free PMC article.
-
The intestinal microbial community and function of Riptortus pedestris at different developmental stages and its effects on development.Front Microbiol. 2025 Jan 22;16:1517280. doi: 10.3389/fmicb.2025.1517280. eCollection 2025. Front Microbiol. 2025. PMID: 39935633 Free PMC article.
-
Mosquito Gut Microbiota: A Review.Pathogens. 2024 Aug 15;13(8):691. doi: 10.3390/pathogens13080691. Pathogens. 2024. PMID: 39204291 Free PMC article. Review.
-
Advances in the dissection of Anopheles-Plasmodium interactions.PLoS Pathog. 2025 Mar 31;21(3):e1012965. doi: 10.1371/journal.ppat.1012965. eCollection 2025 Mar. PLoS Pathog. 2025. PMID: 40163471 Free PMC article. Review.
-
Host complement C3 promotes malaria transmission by killing symbiotic bacteria in the mosquito midgut.Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2424570122. doi: 10.1073/pnas.2424570122. Epub 2025 May 28. Proc Natl Acad Sci U S A. 2025. PMID: 40434644
References
-
- World Malaria Report (World Health Organization, 2019).
-
- Global Vector Control Response 2017–2030 (World Health Organization, 2017).
-
- Dondorp AM et al. Artemisinin resistance: current status and scenarios for containment. Nat. Rev. Microbiol. 8, 272–280 (2010). - PubMed
-
- Ranson H & Lissenden N Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196 (2016). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

