Development of a Scalable and Sublimation-Free Route to MTAD
- PMID: 33958851
- PMCID: PMC8096181
- DOI: 10.1021/acs.oprd.0c00470
Development of a Scalable and Sublimation-Free Route to MTAD
Abstract
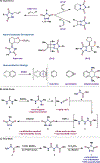
The cyclic azodicarbonyl 4-methyl-1,2,4-triazoline-3,5-dione (MTAD) is a versatile and powerful reagent used mainly in cycloaddition chemistry. Though known for more than 50 years, its unsafe preparation, as well as purification by sublimation, hampered its widespread applicability on a larger scale. Herein we report a scalable and safe route to MTAD, which avoids the generation of methyl isocyanate. Moreover, we demonstrate that sublimation can be circumvented by the application of judicious oxidation conditions, followed by simple filtration. Overall, up to 25 g of MTAD was prepared in a single batch from commercial starting materials in three steps, with recrystallization serving as the only purification in the sequence. When employed in dearomative methodologies, the MTAD obtained by this protocol displayed synthetic efficiency equivalent to that of MTAD purified by sublimation.
Keywords: 1,2,4-triazoline-3,5-dione; MTAD; arenophile; dienophile; urazole.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Formation of Isolable Dearomatized [4 + 2] Cycloadducts from Benzenes, Naphthalenes, and N-Heterocycles Using 1,2-Dihydro-1,2,4,5-tetrazine-3,6-diones as Arenophiles under Visible Light Irradiation.J Am Chem Soc. 2023 Apr 26;145(16):9326-9333. doi: 10.1021/jacs.3c02556. Epub 2023 Apr 13. J Am Chem Soc. 2023. PMID: 37055373
-
A novel pathway in the photooxygenation of cyclic allenes.Org Lett. 2000 May 18;2(10):1383-5. doi: 10.1021/ol005692a. Org Lett. 2000. PMID: 10814453
-
Water-Involved Ring-Opening of 4-Phenyl-1,2,4-triazoline-3,5-dione for "Photo-Clicked" Access to Carbamoyl Formazan Photoswitches In Situ.Chem Asian J. 2022 Jan 17;17(2):e202101239. doi: 10.1002/asia.202101239. Epub 2021 Dec 16. Chem Asian J. 2022. PMID: 34851039
-
Mixture tetracycline citric acid and detergent - A root canal irrigant. A review.J Oral Biol Craniofac Res. 2013 Jan-Apr;3(1):31-5. doi: 10.1016/j.jobcr.2012.09.001. Epub 2012 Sep 14. J Oral Biol Craniofac Res. 2013. PMID: 25737877 Free PMC article. Review.
-
Comparative Evaluation of Antimicrobial Efficacy of MTAD, 3% NaOCI and Propolis Against E Faecalis.Int J Clin Pediatr Dent. 2010 Jan-Apr;3(1):21-5. doi: 10.5005/jp-journals-10005-1049. Epub 2010 Apr 15. Int J Clin Pediatr Dent. 2010. PMID: 27625552 Free PMC article. Review.
Cited by
-
Telescoped Oxidation and Cycloaddition of Urazoles to Access Diazacyclobutenes.J Org Chem. 2022 Jun 3;87(11):7494-7500. doi: 10.1021/acs.joc.2c00280. Epub 2022 May 12. J Org Chem. 2022. PMID: 35549283 Free PMC article.
-
Palladium-catalyzed dearomative 1,4-hydroamination.Tetrahedron. 2024 Aug 21;163:134135. doi: 10.1016/j.tet.2024.134135. Epub 2024 Jul 4. Tetrahedron. 2024. PMID: 40919097 Free PMC article.
References
-
- De Bruycker K; Billiet S; Houck HA; Chattopadhyay S; Winne JM; Du Prez FE Triazolinediones as Highly Enabling Synthetic Tools. Chem. Rev. 2016, 116 (6), 3919–3974. - PubMed
- Levek TJ; Kiefer EF The Mechanism of Allene Cycloaddition. III. Thermal and Photochemical Generation of 2,2’-Bis(1,1-Dimethylallyl) Biradical from an Azocyclane Precursor. J. Am. Chem. Soc. 1976, 98 (7), 1875–1879.
- Dowd P; Weber W Preparation and Diels-Alder Reaction of (1E)-1,3-Dimethoxybutadiene. J. Org. Chem. 1982, 47 (24), 4774–4777.
- (d) Boan C; Skattebøl L Cycloadditions to Conjugated Diallenes. J. Chem. Soc., Perkin Trans. 1 1978, 12, 1568–1572.
- Ando W; Hanyu Y; Takata T Six-Membered Ring-Fused Thiirene S-Oxides. Synthesis, Characterization, and Reactivity. J. Org. Chem. 1986, 51 (11), 2122–2125.
- Clive DLJ; Bergstra RJ A Route to Linear, Bridged, or Spiro Polycyclic Compounds: Sequential Use of the Intermolecular Diels-Alder Reaction and Radical Cyclization. J. Org. Chem. 1990, 55 (6), 1786–1792.
-
- Breton GW; Shugart JH; Hughey CA; Perala SM; Hicks AD Synthesis of Δ1–1,2-Diazetines via a Diels–Alder Cycloaddition Approach. Org. Lett. 2001, 3 (20), 3185–3187. - PubMed
- Seymour CA; Greene FD Mechanism of Triazolinedione-Olefin Reactions. Ene and Cycloaddition. J. Am. Chem. Soc. 1980, 102 (20), 6384–6385.
- Hall JH; Krishnan G The 2+2 Cycloaddition of 4-Substituted-1,2,4-Triazoline-3,5-Diones to Diphenylketene. J. Org. Chem. 1984, 49 (13), 2498–2500.
-
- Pirkle WH; Stickler JC The Reaction of 1,2,4-Triazoline-3,5-Diones with Mono-Olefins. Chem. Commun. (London) 1967, 15, 760–761.
- Orfanopoulos M; Elemes Y; Stratakis M Reactions of Triazolinedione with Alkenes. A Remarkable Geminal Selectivity. Tetrahedron Lett. 1990, 31 (40), 5775–5778.
- Watson LJ; Harrington RW; Clegg W; Hall MJ Diastereoselective Intermolecular Ene Reactions: Synthesis of 4,5,6,7-Tetrahydro-1H-Benzo[d]Imidazoles. Org. Biomol. Chem. 2012, 10 (33), 6649–6655. - PubMed
- Pastor A; Adam W; Wirth T; Tóth G. Diastereoselective Reactions of the Tiglic Acid Functionality Mediated by Oxazolidine Chiral Auxiliaries: A Mechanistic Comparison of DMD Andm-CPBA Epoxidations versus Singlet Oxygen and PTAD Ene Reactions. Eur. J. Org. Chem. 2005, 2005 (14), 3075–3084.
- Gau A-H; Lin G-L; Uang B-J; Liao F-L; Wang S-L Regio- and Diastereoselective Ene Reaction of 4-Phenyl-1,2,4-Triazoline-3,5-Dione with Chiral Allylic Alcohols and Their Derivatives. J. Org. Chem. 1999, 64 (7), 2194–2201.
-
- Ban H; Nagano M; Gavrilyuk J; Hakamata W; Inokuma T; Barbas CF Facile and Stabile Linkages through Tyrosine: Bioconjugation Strategies with the Tyrosine-Click Reaction. Bioconjugate Chem. 2013, 24 (4), 520–532. - PMC - PubMed
- Ban H; Gavrilyuk J; Barbas CF Tyrosine Bioconjugation through Aqueous Ene-Type Reactions: A Click-Like Reaction for Tyrosine. J. Am. Chem. Soc. 2010, 132 (5), 1523–1525. - PubMed
-
- Mondal P; Behera PK; Singha NK A Healable Thermo-Reversible Functional Polymer Prepared via RAFT Polymerization and Ultrafast ‘Click’ Chemistry Using a Triazolinedione Derivative. Chem. Commun. 2017, 53 (62), 8715–8718. - PubMed
- Defize T; Riva R; Thomassin J-M; Alexandre M; Herck NV; Prez FD; Jérôme C Reversible TAD Chemistry as a Convenient Tool for the Design of (Re)Processable PCL-Based Shape-Memory Materials. Macromol. Rapid Commun. 2017, 38 (1), 1600517. - PubMed
- Mondal P; Raut SK; Singha NK Thermally Amendable Tailor-Made Acrylate Copolymers via RAFT Polymerization and Ultrafast Alder-Ene “Click” Chemistry. J. Polym. Sci., Part A: Polym. Chem. 2018, 56 (20), 2310–2318.
Grants and funding
LinkOut - more resources
Full Text Sources
