In silico Gene Set and Pathway Enrichment Analyses Highlight Involvement of Ion Transport in Cholinergic Pathways in Autism: Rationale for Nutritional Intervention
- PMID: 33958984
- PMCID: PMC8093449
- DOI: 10.3389/fnins.2021.648410
In silico Gene Set and Pathway Enrichment Analyses Highlight Involvement of Ion Transport in Cholinergic Pathways in Autism: Rationale for Nutritional Intervention
Abstract
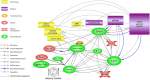
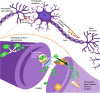
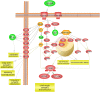
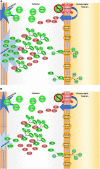
Food is the primary human source of choline, an essential precursor to the neurotransmitter acetylcholine, which has a central role in signaling pathways that govern sensorimotor functions. Most Americans do not consume their recommended amount of dietary choline, and populations with neurodevelopmental conditions like autism spectrum disorder (ASD) may be particularly vulnerable to consequences of choline deficiency. This study aimed to identify a relationship between ASD and cholinergic signaling through gene set enrichment analysis and interrogation of existing database evidence to produce a systems biology model. In gene set enrichment analysis, two gene ontologies were identified as overlapping for autism-related and for cholinergic pathways-related functions, both involving ion transport regulation. Subsequent modeling of ion transport intensive cholinergic signaling pathways highlighted the importance of two genes with autism-associated variants: GABBR1, which codes for the gamma aminobutyric acid receptor (GABAB 1), and KCNN2, which codes for calcium-activated, potassium ion transporting SK2 channels responsible for membrane repolarization after cholinergic binding/signal transmission events. Cholinergic signal transmission pathways related to these proteins were examined in the Pathway Studio environment. The ion transport ontological associations indicated feasibility of a dietary choline support as a low-risk therapeutic intervention capable of modulating cholinergic sensory signaling in autism. Further research at the intersection of dietary status and sensory function in autism is warranted.
Keywords: acetylcholine; autism; cholinergic; dietary choline; gene set enrichment analysis; sensory processing.
Copyright © 2021 Olson, Zhang, Cao, Baranova and Slavin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
An Evolving Therapeutic Rationale for Targeting the α7 Nicotinic Acetylcholine Receptor in Autism Spectrum Disorder.Curr Top Behav Neurosci. 2020;45:167-208. doi: 10.1007/7854_2020_136. Curr Top Behav Neurosci. 2020. PMID: 32468495
-
Pathway Network Analyses for Autism Reveal Multisystem Involvement, Major Overlaps with Other Diseases and Convergence upon MAPK and Calcium Signaling.PLoS One. 2016 Apr 7;11(4):e0153329. doi: 10.1371/journal.pone.0153329. eCollection 2016. PLoS One. 2016. PMID: 27055244 Free PMC article.
-
Ionic Channels as Potential Targets for the Treatment of Autism Spectrum Disorder: A Review.Curr Neuropharmacol. 2022;20(10):1834-1849. doi: 10.2174/1570159X19666210809102547. Curr Neuropharmacol. 2022. PMID: 34370640 Free PMC article. Review.
-
Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. I. Central nervous system.Neuroscience. 1998 May;84(2):331-59. doi: 10.1016/s0306-4522(97)00516-2. Neuroscience. 1998. PMID: 9539209
-
Modulation of cholinergic systems by manganese.Neurotoxicology. 2007 Sep;28(5):1003-14. doi: 10.1016/j.neuro.2007.08.006. Epub 2007 Aug 22. Neurotoxicology. 2007. PMID: 17920128 Review.
Cited by
-
Prevalence, Socio-Demographic Characteristics, and Co-Morbidities of Autism Spectrum Disorder in US Children: Insights from the 2020-2021 National Survey of Children's Health.Children (Basel). 2025 Feb 27;12(3):297. doi: 10.3390/children12030297. Children (Basel). 2025. PMID: 40150580 Free PMC article.
-
Computational Neuroscience's Influence on Autism Neuro-Transmission Research: Mapping Serotonin, Dopamine, GABA, and Glutamate.Biomedicines. 2025 Jun 10;13(6):1420. doi: 10.3390/biomedicines13061420. Biomedicines. 2025. PMID: 40564138 Free PMC article. Review.
-
Mitochondrial Disruption by Amyloid Beta 42 Identified by Proteomics and Pathway Mapping.Cells. 2021 Sep 10;10(9):2380. doi: 10.3390/cells10092380. Cells. 2021. PMID: 34572029 Free PMC article.
-
Nutritional Intake and Sensory Processing in School-Aged Children with Autism Spectrum Disorder.Nutrients. 2025 Feb 7;17(4):604. doi: 10.3390/nu17040604. Nutrients. 2025. PMID: 40004933 Free PMC article.
References
-
- Ashburner J., Ziviani J., Rodger S. (2008). Sensory processing and classroom emotional, behavioral, and educational outcomes in children with autism spectrum disorder. Am. J. Occup. Ther. Bethesda 62 564–573. - PubMed
-
- Bahia P. K., Suzuki R., Benton D. C. H., Jowett A. J., Chen M. X., Trezise D. J., et al. (2005). A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes. J. Neurosci. 25 3489–3498. 10.1523/JNEUROSCI.0597-05.2005 - DOI - PMC - PubMed
-
- Baranek G. T., David F. J., Poe M. D., Stone W. L., Watson L. R. (2006). Sensory experiences questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry 47 591–601. 10.1111/j.1469-7610.2005.01546.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

