Evolving Roles of Muscle-Resident Fibro-Adipogenic Progenitors in Health, Regeneration, Neuromuscular Disorders, and Aging
- PMID: 33959042
- PMCID: PMC8093402
- DOI: 10.3389/fphys.2021.673404
Evolving Roles of Muscle-Resident Fibro-Adipogenic Progenitors in Health, Regeneration, Neuromuscular Disorders, and Aging
Abstract
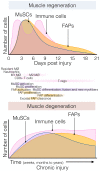
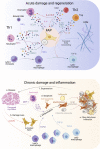
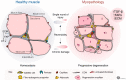
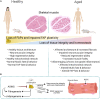
Normal skeletal muscle functions are affected following trauma, chronic diseases, inherited neuromuscular disorders, aging, and cachexia, hampering the daily activities and quality of life of the affected patients. The maladaptive accumulation of fibrous intramuscular connective tissue and fat are hallmarks of multiple pathologies where chronic damage and inflammation are not resolved, leading to progressive muscle replacement and tissue degeneration. Muscle-resident fibro-adipogenic progenitors are adaptable stromal cells with multilineage potential. They are required for muscle homeostasis, neuromuscular integrity, and tissue regeneration. Fibro-adipogenic progenitors actively regulate and shape the extracellular matrix and exert immunomodulatory functions via cross-talk with multiple other residents and non-resident muscle cells. Remarkably, cumulative evidence shows that a significant proportion of activated fibroblasts, adipocytes, and bone-cartilage cells, found after muscle trauma and disease, descend from these enigmatic interstitial progenitors. Despite the profound impact of muscle disease on human health, the fibrous, fatty, and ectopic bone tissues' origins are poorly understood. Here, we review the current knowledge of fibro-adipogenic progenitor function on muscle homeostatic integrity, regeneration, repair, and aging. We also discuss how scar-forming pathologies and disorders lead to dysregulations in their behavior and plasticity and how these stromal cells can control the onset and severity of muscle loss in disease. We finally explore the rationale of improving muscle regeneration by understanding and modulating fibro-adipogenic progenitors' fate and behavior.
Keywords: aging; duchenne muscular dystrophy (DMD); extracellular matrix (ECM); macrophages; muscle FAPs; muscle regeneration; muscle stem cells (MuSCs); skeletal muscle fibrosis.
Copyright © 2021 Theret, Rossi and Contreras.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abramowitz M. K., Paredes W., Zhang K., Brightwell C. R., Newsom J. N., Kwon H. J., et al. (2018). Skeletal muscle fibrosis is associated with decreased muscle inflammation and weakness in patients with chronic kidney disease. Am. J. Physiol. Ren. Physiol. 315 F1658–F1669. 10.1152/ajprenal.00314.2018 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

