Melatonin: Multi-Target Mechanism Against Diminished Ovarian Reserve Based on Network Pharmacology
- PMID: 33959095
- PMCID: PMC8095380
- DOI: 10.3389/fendo.2021.630504
Melatonin: Multi-Target Mechanism Against Diminished Ovarian Reserve Based on Network Pharmacology
Abstract
Background: Diminished ovarian reserve (DOR) significantly increases the risk of female infertility and contributes to reproductive technology failure. Recently, the role of melatonin in improving ovarian reserve (OR) has attracted widespread attention. However, details on the pharmacological targets and mechanisms of melatonin-improved OR remain unclear.
Objective: A systems pharmacology strategy was proposed to elucidate the potential therapeutic mechanism of melatonin on DOR at the molecular, pathway, and network levels.
Methods: The systems pharmacological approach consisted of target identification, data integration, network construction, bioinformatics analysis, and molecular docking.
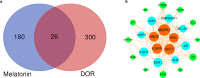
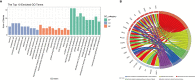
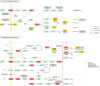
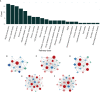
Results: From the molecular perspective, 26 potential therapeutic targets were identified. They participate in biological processes related to DOR development, such as reproductive structure development, epithelial cell proliferation, extrinsic apoptotic signaling pathway, PI3K signaling, among others. Eight hub targets (MAPK1, AKT1, EGFR, HRAS, SRC, ESR1, AR, and ALB) were identified. From the pathway level, 17 significant pathways, including the PI3K-Akt signaling pathway and the estrogen signaling pathway, were identified. In addition, the 17 signaling pathways interacted with the 26 potential therapeutic targets to form 4 functional modules. From the network point of view, by regulating five target subnetworks (aging, cell growth and death, development and regeneration, endocrine and immune systems), melatonin could exhibit anti-aging, anti-apoptosis, endocrine, and immune system regulation effects. The molecular docking results showed that melatonin bound well to all hub targets.
Conclusion: This study systematically and intuitively illustrated the possible pharmacological mechanisms of OR improvement by melatonin through anti-aging, anti-apoptosis, endocrine, and immune system regulation effects.
Keywords: biological processes; diminished ovarian reserve (DOR); melatonin; network pharmacology; ovarian reserve (OR); potential therapeutic targets; signaling pathways.
Copyright © 2021 Yang, Xu, Chen, Miao, Zhao, Xing and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Potential mechanism prediction of indole-3-propionic acid against diminished ovarian reserve via network pharmacology, molecular docking and experimental verification.BMC Complement Med Ther. 2024 Aug 27;24(1):316. doi: 10.1186/s12906-024-04611-1. BMC Complement Med Ther. 2024. PMID: 39192219 Free PMC article.
-
Investigation of the Mechanisms and Experimental Verification of Yulin Formula in the Treatment of Diminished Ovarian Reserve via Network Pharmacology.Drug Des Devel Ther. 2023 Jul 24;17:2147-2163. doi: 10.2147/DDDT.S413142. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37521037 Free PMC article.
-
The Network Pharmacology Analysis of Tonifying Yang Formula for the Treatment of Diminished Ovarian Reserve.Altern Ther Health Med. 2024 Mar;30(3):176-184. Altern Ther Health Med. 2024. PMID: 37883761
-
[Research progress of acupuncture for the improvement of ovarian reserve by regulating different signal pathways].Zhen Ci Yan Jiu. 2022 Jul 25;47(7):644-8. doi: 10.13702/j.1000-0607.20210330. Zhen Ci Yan Jiu. 2022. PMID: 35880284 Review. Chinese.
-
The role of growth hormone in assisted reproductive technology for patients with diminished ovarian reserve: from signaling pathways to clinical applications.Front Endocrinol (Lausanne). 2025 Apr 17;16:1551126. doi: 10.3389/fendo.2025.1551126. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40313485 Free PMC article. Review.
Cited by
-
Potential mechanism prediction of indole-3-propionic acid against diminished ovarian reserve via network pharmacology, molecular docking and experimental verification.BMC Complement Med Ther. 2024 Aug 27;24(1):316. doi: 10.1186/s12906-024-04611-1. BMC Complement Med Ther. 2024. PMID: 39192219 Free PMC article.
-
Therapeutic Target Analysis and Molecular Mechanism of Melatonin - Treated Leptin Resistance Induced Obesity: A Systematic Study of Network Pharmacology.Front Endocrinol (Lausanne). 2022 Jul 22;13:927576. doi: 10.3389/fendo.2022.927576. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35937803 Free PMC article.
-
Use of Network Pharmacology and Experiment Validation to Uncover the Mechanism of Jianshen Lishui Prescription in the Treatment of Intracerebral Hemorrhage.Comb Chem High Throughput Screen. 2025;28(3):453-466. doi: 10.2174/0113862073256436231031100059. Comb Chem High Throughput Screen. 2025. PMID: 38243958
-
Causal relationship between fertility nutrients supplementation and PCOS risk: a Mendelian randomization study.Front Endocrinol (Lausanne). 2024 Sep 24;15:1420004. doi: 10.3389/fendo.2024.1420004. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39381438 Free PMC article.
-
The therapeutic role and potential mechanism of EGCG in obesity-related precocious puberty as determined by integrated metabolomics and network pharmacology.Front Endocrinol (Lausanne). 2023 Jun 2;14:1159657. doi: 10.3389/fendo.2023.1159657. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37334310 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

