Development of Vasoinhibin-Specific Monoclonal Antibodies
- PMID: 33959096
- PMCID: PMC8095375
- DOI: 10.3389/fendo.2021.645085
Development of Vasoinhibin-Specific Monoclonal Antibodies
Abstract
Vasoinhibin is a protein hormone with antiangiogenic, antivasodilatatory, and antivasopermeability effects generated by the proteolytic cleavage of prolactin. The discovery of its role in diabetic retinopathy and peripartum cardiomyopathy led to the evaluation of new pharmacological treatments in clinical interventional trials. However, the quantitative evaluation of vasoinhibin in biological samples from patients has not been possible due to the lack of vasoinhibin-specific antibodies. Recently, loop 1 of vasoinhibin was identified to have a different three-dimensional structure compared to PRL, and thus to contain vasoinhibin-specific epitopes. Here, we report the development of two sets of vasoinhibin-specific monoclonal antibodies against two neighboring regions of the vasoinhibin loop 1. An experimental sandwich ELISA with two monoclonal anti-vasoinhibin antibodies was developed, which had no cross-reactivity to recombinant human full-length prolactin. The ELISA had a quantitation limit of 100 ng/ml, and intra-assay- and inter-assay coefficients of variation of 12.5% and 14%, respectively. The evaluation of 15 human serum samples demonstrated concentrations of below limit of detection (n=3), below limit of quantitation (n=1) and between 0.23 µg/ml (230 ng/ml) to 605 µg/ml (n=12) in the quantifiable range. Despite the high specificity of the monoclonal-monoclonal antibody sandwiches which discriminate vasoinhibin from PRL, there might be cross-reactivities by serum proteins other than vasoinhibin. A fully established vasoinhibin ELISA may support diagnostic and therapeutic measures in vascular diseases.
Keywords: 16K PRL; ELISA - enzyme-linked immunosorbent assay; monoclonal antibodies; prolactin (PRL); vasoinhibin.
Copyright © 2021 Müller, Robles, Zamora, Ebnet, Markl-Hahn, Martínez de la Escalera, Clapp, Bertsch and Triebel.
Conflict of interest statement
The authors declare the following competing interests: CC, JPR, MZ, GME, JT, and TB are inventors of a pending Mexican (MX/E/2019/079075) and International (PCT/EP2020/069154) patent application covering the use of the anti-vasoinhibin monoclonal antibodies for diagnostic purposes. The Universidad Nacional Autónoma de México (UNAM), and the authors JT and TB are owners of the pending-patent applications. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

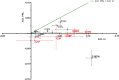
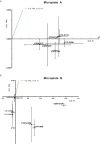
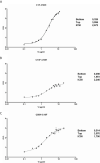
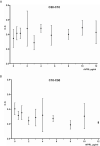
References
-
- Cruz-Soto ME, Cosio G, Jeziorski MC, Vargas-Barroso V, Aguilar MB, Carabez A, et al. . Cathepsin D is the primary protease for the generation of adenohypophyseal vasoinhibins: cleavage occurs within the prolactin secretory granules. Endocrinology (2009) 150(12):5446–54. 10.1210/en.2009-0390 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

