Metabolic Alterations in Shrimp Stomach During Acute Hepatopancreatic Necrosis Disease and Effects of Taurocholate on Vibrio parahaemolyticus
- PMID: 33959104
- PMCID: PMC8093816
- DOI: 10.3389/fmicb.2021.631468
Metabolic Alterations in Shrimp Stomach During Acute Hepatopancreatic Necrosis Disease and Effects of Taurocholate on Vibrio parahaemolyticus
Abstract
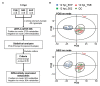
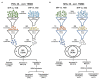
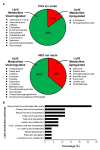
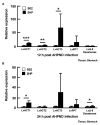
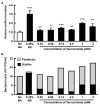
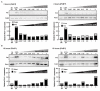
Acute hepatopancreatic necrosis disease (AHPND), a recently emerged bacterial shrimp disease, has increased shrimp mortality and caused huge economic losses in many Asian countries. However, molecular factors underlying pathogenesis of this disease remain largely unknown. Our objective was to characterize metabolic alterations in shrimp stomach during AHPND and determine effects of taurocholate on AHPND-causing Vibrio parahaemolyticus. Based on metabolomics, pathways for lipid metabolism and for primary bile acid (BA) synthesis were majorly affected following AHPND infection. Bile acid metabolites, namely taurocholate, were downregulated in the metabolomics database. This prompted us to study effects of taurocholate on biofilm formation, PirAB vp toxin release and biofilm detachment capabilities in AHPND-causing V. parahaemolyticus. Treatment of this bacterium with high concentration of taurocholate, a primary bile acid, induced biofilm formation, PirAB vp toxin release and facilitated the dispersion of bacterial cells. Taken together, our findings suggest that AHPND infection can affect the lipid metabolites in shrimp stomach, and further suggest that the primary bile acid taurocholate is important for the virulence of AHPND-causing V. parahaemolyticus.
Keywords: AHPND; Vibrio parahaemolyticus; lipid metabolism; metabolomics; shrimp; taurocholate.
Copyright © 2021 Kumar, Tung, Ng, Chang, Chen, Chen, Lin and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

