Understanding the Sorghum- Colletotrichum sublineola Interactions for Enhanced Host Resistance
- PMID: 33959139
- PMCID: PMC8093437
- DOI: 10.3389/fpls.2021.641969
Understanding the Sorghum- Colletotrichum sublineola Interactions for Enhanced Host Resistance
Abstract
Improving sorghum resistance is a sustainable method to reduce yield losses due to anthracnose, a devastating disease caused by Colletotrichum sublineola. Elucidating the molecular mechanisms of sorghum-C. sublineola interactions would help identify biomarkers for rapid and efficient identification of novel sources for host-plant resistance improvement, understanding the pathogen virulence, and facilitating resistance breeding. Despite concerted efforts to identify resistance sources, the knowledge about sorghum-anthracnose interactions remains scanty. Hence, in this review, we presented an overview of the current knowledge on the mechanisms of sorghum-C. sublineola molecular interactions, sources of resistance for sorghum breeding, quantitative trait loci (QTL), and major (R-) resistance gene sequences as well as defense-related genes associated with anthracnose resistance. We summarized current knowledge about C. sublineola populations and its virulence. Illustration of the sorghum-C. sublineola interaction model based on the current understanding is also provided. We highlighted the importance of genomic resources of both organisms for integrated omics research to unravel the key molecular components underpinning compatible and incompatible sorghum-anthracnose interactions. Furthermore, sorghum-breeding strategy employing rapid sorghum germplasm screening, systems biology, and molecular tools is presented.
Keywords: Colletotrichum sublineola; R-genes; anthracnose; germplasm; host-plant resistance; quantitative trait loci; sorghum.
Copyright © 2021 Abreha, Ortiz, Carlsson and Geleta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
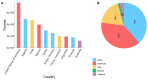
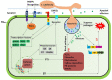

References
-
- Afolayan G., Deshpande S. P., Aladele S. E., Kolawole A. O., Angarawai I., Nwosu D. J., et al. . (2019). Genetic diversity assessment of sorghum (Sorghum bicolor (L.) Moench) accessions using single nucleotide polymorphism markers. Plant Genet. Resour. 17, 412–420. 10.1017/S1479262119000212 - DOI
-
- Ahn E., Prom L. K., Odvody G., Magill C. (2019). Defense responses against the sorghum anthracnose pathogen in leaf blade and midrib tissue of johnsongrass and sorghum. Physiol. Mol. Plant Pathol. 106, 81–86. 10.1016/j.pmpp.2018.12.008 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

