Patient-reported outcomes from a randomized, double-blind, placebo controlled, phase III study of baricitinib versus placebo in patients with moderately to severely active rheumatoid arthritis and an inadequate response to methotrexate therapy: results from the RA-BALANCE study
- PMID: 33959198
- PMCID: PMC8064513
- DOI: 10.1177/1759720X211006964
Patient-reported outcomes from a randomized, double-blind, placebo controlled, phase III study of baricitinib versus placebo in patients with moderately to severely active rheumatoid arthritis and an inadequate response to methotrexate therapy: results from the RA-BALANCE study
Abstract
Introduction: To assess the effect of baricitinib on patient-reported outcomes (PROs) in patients with moderately to severely active rheumatoid arthritis (RA) who had an inadequate response to methotrexate (MTX).
Methods: This was a 52-week, randomized, double-blind, placebo controlled, phase III study in patients with RA who had an inadequate response to MTX. Patients (n = 290) receiving stable background MTX were randomly assigned (1:1) to receive placebo or baricitinib 4 mg once daily with a primary endpoint at week 12. PROs assessed included Health Assessment Questionnaire-Disability Index (HAQ-DI), Patient's Global Assessment of Disease Activity, patient's assessment of pain, Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), European Quality of Life-5 Dimensions-5 Level index scores and visual analogue scale, and measures collected in electronic patient daily diaries: duration of morning joint stiffness, Worst Tiredness, and Worst Joint Pain. Treatment comparisons were made with logistic regression and analysis of covariance models for categorical and continuous variables, respectively.
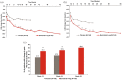
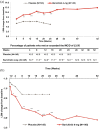
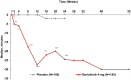
Results: Statistically significant (p ⩽ 0.05) improvements in all PROs were observed in the baricitinib 4 mg group compared to placebo as early as week 1 to week 4; and were sustained to week 24. These improvements were maintained until week 52 for the baricitinib group. A significantly larger proportion of patients met or exceeded the minimum clinically important difference for HAQ-DI (⩾0.22) and FACIT-F (3.56) profiles in the baricitinib group.
Conclusion: Baricitinib provided significant improvements in PROs compared to placebo to 52 weeks of treatment in patients with RA who had an inadequate response to MTX.Clinicaltrials.gov identifier: https://clinicaltrials.gov/ct2/show/NCT02265705; NCT02265705; RA-BALANCE. Registered 13 October 2014.
Keywords: China; baricitinib; inadequate response; methotrexate; patient-reported outcomes; rheumatoid arthritis.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: Yue Yang, Jianhua Xu, Jian Xu, Xingfu Li, Jiankang Hu, Xiangpei Li, Xiao Zhang, Dongyi He, Zhijun Li, Alberto J Spindler and Zhanguo Li have nothing to disclose. Chunde Bao and Guochun Wang serve as advisory board members for baricitinib and have received consulting and/or speaking fees from Eli Lilly and Company (<$10,000 USD). Cristiano AF Zerbini reports grants from Eli Lilly and Company, Pfizer, Sanofi, and AbbVie outside the submitted work. Carol L Kannowski is a full-time employee of Eli Lilly and Company, Indianapolis, USA; reports personal fees from Eli Lilly and Company outside the submitted work; and is a stockholder in Eli Lilly and Company. The authors Lujing Zhan and Mengru Liu are full-time employees of Lilly Suzhou Pharmaceutical Co. Ltd., Shanghai, China. The authors Hanjun Wu and Fei Ji are full-time employees of Lilly Suzhou Pharmaceutical Co. Ltd., Shanghai, China and own stock in Eli Lilly and Company.
Figures


References
-
- Kiltz U, van der Heijde D. Health-related quality of life in patients with rheumatoid arthritis and in patients with ankylosing spondylitis. Clin Exp Rheumatol 2009; 27 (Suppl. 55): S108–S111. - PubMed
-
- Cross M, Smith E, Hoy D, et al.. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73: 1316–1322. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

