Doxycycline Hyclate Modulates Antioxidant Defenses, Matrix Metalloproteinases, and COX-2 Activity Accelerating Skin Wound Healing by Secondary Intention in Rats
- PMID: 33959214
- PMCID: PMC8075706
- DOI: 10.1155/2021/4681041
Doxycycline Hyclate Modulates Antioxidant Defenses, Matrix Metalloproteinases, and COX-2 Activity Accelerating Skin Wound Healing by Secondary Intention in Rats
Abstract
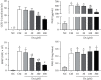
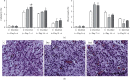
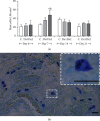
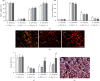
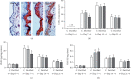
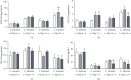
The main objective of this study was to investigate the action of doxycycline hyclate (Dx) in the skin wound healing process in Wistar rats. We investigated the effect of Dx on inflammatory cell recruitment and production of inflammatory mediators via in vitro and in vivo analysis. In addition, we analyzed neovascularization, extracellular matrix deposition, and antioxidant potential of Dx on cutaneous repair in Wistar rats. Male animals (n = 15) were divided into three groups with five animals each (protocol: 72/2017), and three skin wounds (12 mm diameter) were created on the back of the animals. The groups were as follows: C, received distilled water (control); Dx1, doxycycline hyclate (10 mg/kg/day); and Dx2, doxycycline hyclate (30 mg/kg/day). The applications were carried out daily for up to 21 days, and tissues from different wounds were removed every 7 days. Our in vitro analysis demonstrated that Dx led to macrophage proliferation and increased N-acetyl-β-D-glucosaminidase (NAG) production, besides decreased cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and metalloproteinases (MMP), which indicates that macrophage activation and COX-2 inhibition are possibly regulated by independent mechanisms. In vivo, our findings presented increased cellularity, blood vessels, and the number of mast cells. However, downregulation was observed in the COX-2 and PGE2 expression, which was limited to epidermal cells. Our results also showed that the downregulation of this pathway benefits the oxidative balance by reducing protein carbonyls, malondialdehyde, nitric oxide, and hydrogen peroxide (H2O2). In addition, there was an increase in the antioxidant enzymes (catalase and superoxide dismutase) after Dx exposure, which demonstrates its antioxidant potential. Finally, Dx increased the number of types I collagen and elastic fibers and reduced the levels of MMP, thus accelerating the closure of skin wounds. Our findings indicated that both doses of Dx can modulate the skin repair process, but the best effects were observed after exposure to the highest dose.
Copyright © 2021 Luciana S. Altoé et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Figures


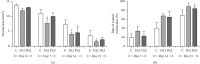

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

