Ketogenic Diet Suppressed T-Regulatory Cells and Promoted Cardiac Fibrosis via Reducing Mitochondria-Associated Membranes and Inhibiting Mitochondrial Function
- PMID: 33959215
- PMCID: PMC8075689
- DOI: 10.1155/2021/5512322
Ketogenic Diet Suppressed T-Regulatory Cells and Promoted Cardiac Fibrosis via Reducing Mitochondria-Associated Membranes and Inhibiting Mitochondrial Function
Abstract
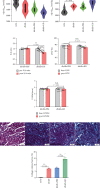
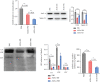
Ketogenic diet (KD) is popular in diabetic patients but its cardiac safety and efficiency on the heart are unknown. The aim of the present study is to determine the effects and the underlined mechanisms of KD on cardiac function in diabetic cardiomyopathy (DCM). We used db/db mice to model DCM, and different diets (regular or KD) were used. Cardiac function and interstitial fibrosis were determined. T-regulatory cell (Treg) number and functions were evaluated. The effects of ketone body (KB) on fatty acid (FA) and glucose metabolism, mitochondria-associated endoplasmic reticulum membranes (MAMs), and mitochondrial respiration were assessed. The mechanisms via which KB regulated MAMs and Tregs were addressed. KD improved metabolic indices in db/db mice. However, KD impaired cardiac diastolic function and exacerbated ventricular fibrosis. Proportions of circulatory CD4+CD25+Foxp3+ cells in whole blood cells and serum levels of IL-4 and IL-10 were reduced in mice fed with KD. KB suppressed the differentiation to Tregs from naive CD4+ T cells. Cultured medium from KB-treated Tregs synergically activated cardiac fibroblasts. Meanwhile, KB inhibited Treg proliferation and productions of IL-4 and IL-10. Treg MAMs, mitochondrial respiration and respiratory complexes, and FA synthesis and oxidation were all suppressed by KB while glycolytic levels were increased. L-carnitine reversed Treg proliferation and function inhibited by KB. Proportions of ST2L+ cells in Tregs were reduced by KB, as well as the production of ST2L ligand, IL-33. Reinforcement expressions of ST2L in Tregs counteracted the reductions in MAMs, mitochondrial respiration, and Treg proliferations and productions of Treg cytokines IL-4 and IL-10. Therefore, despite the improvement of metabolic indices, KD impaired Treg expansion and function and promoted cardiac fibroblast activation and interstitial fibrosis. This could be mainly mediated by the suppression of MAMs and fatty acid metabolism inhibition via blunting IL-33/ST2L signaling.
Copyright © 2021 Jun Tao et al.
Conflict of interest statement
All authors declare no potential conflict of interest.
Figures



Similar articles
-
Ketogenic diet modulates cardiac metabolic dysregulation in streptozocin-induced diabetic rats.J Nutr Biochem. 2023 Jan;111:109161. doi: 10.1016/j.jnutbio.2022.109161. Epub 2022 Sep 29. J Nutr Biochem. 2023. PMID: 36184012
-
Hyperglycemia-Driven Inhibition of AMP-Activated Protein Kinase α2 Induces Diabetic Cardiomyopathy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes In Vivo.Circulation. 2019 Apr 16;139(16):1913-1936. doi: 10.1161/CIRCULATIONAHA.118.033552. Circulation. 2019. PMID: 30646747 Free PMC article.
-
[Proliferation of CD4+ CD25+ regulatory T cells of rat by different cytokines in vitro].Zhonghua Yi Xue Za Zhi. 2008 Mar 25;88(12):844-7. Zhonghua Yi Xue Za Zhi. 2008. PMID: 18756991 Chinese.
-
Alloantigen specific T regulatory cells in transplant tolerance.Int Immunopharmacol. 2009 May;9(5):570-4. doi: 10.1016/j.intimp.2009.01.016. Epub 2009 Jan 29. Int Immunopharmacol. 2009. PMID: 19539571 Review.
-
Mitochondrial mechanisms in Treg cell regulation: Implications for immunotherapy and disease treatment.Mitochondrion. 2025 Jan;80:101975. doi: 10.1016/j.mito.2024.101975. Epub 2024 Nov 2. Mitochondrion. 2025. PMID: 39491776 Review.
Cited by
-
Regulatory T Cells in Pathological Cardiac Hypertrophy: Mechanisms and Therapeutic Potential.Cardiovasc Drugs Ther. 2024 Oct;38(5):999-1015. doi: 10.1007/s10557-023-07463-y. Epub 2023 May 15. Cardiovasc Drugs Ther. 2024. PMID: 37184744 Review.
-
Mitochondria-associated endoplasmic reticulum membranes as a therapeutic target for cardiovascular diseases.Front Pharmacol. 2024 Apr 17;15:1398381. doi: 10.3389/fphar.2024.1398381. eCollection 2024. Front Pharmacol. 2024. PMID: 38694924 Free PMC article. Review.
-
So close, yet so far away: the relationship between MAM and cardiac disease.Front Cardiovasc Med. 2024 Feb 5;11:1353533. doi: 10.3389/fcvm.2024.1353533. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38374992 Free PMC article. Review.
-
Ketogenic diet induces p53-dependent cellular senescence in multiple organs.Sci Adv. 2024 May 17;10(20):eado1463. doi: 10.1126/sciadv.ado1463. Epub 2024 May 17. Sci Adv. 2024. PMID: 38758782 Free PMC article.
-
Mitochondria-Associated Endoplasmic Reticulum Membranes in Human Health and Diseases.MedComm (2020). 2025 Jun 27;6(7):e70259. doi: 10.1002/mco2.70259. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40584408 Free PMC article. Review.
References
-
- Gao J.-W., Hao Q.-Y., Zhang H.-F., et al. Low-carbohydrate diet score and coronary artery calcium progression: results from the CARDIA Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2020 [cited 2020 Oct 29]; Available from: https://www.ahajournals.org/doi/10.1161/ATVBAHA.120.314838. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

