Pharmacoepidemiology for nephrologists (part 2): potential biases and how to overcome them
- PMID: 33959262
- PMCID: PMC8087121
- DOI: 10.1093/ckj/sfaa242
Pharmacoepidemiology for nephrologists (part 2): potential biases and how to overcome them
Abstract
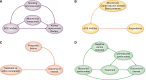
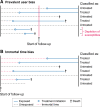
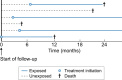
Observational pharmacoepidemiological studies using routinely collected healthcare data are increasingly being used in the field of nephrology to answer questions on the effectiveness and safety of medications. This review discusses a number of biases that may arise in such studies and proposes solutions to minimize them during the design or statistical analysis phase. We first describe designs to handle confounding by indication (e.g. active comparator design) and methods to investigate the influence of unmeasured confounding, such as the E-value, the use of negative control outcomes and control cohorts. We next discuss prevalent user and immortal time biases in pharmacoepidemiology research and how these can be prevented by focussing on incident users and applying either landmarking, using a time-varying exposure, or the cloning, censoring and weighting method. Lastly, we briefly discuss the common issues with missing data and misclassification bias. When these biases are properly accounted for, pharmacoepidemiological observational studies can provide valuable information for clinical practice.
Keywords: bias; causal inference; confounding; epidemiologic methods; observational studies; pharmacoepidemiology.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA.
Figures


References
-
- Begaud B. Dictionary of Pharmacoepidemiology. Hoboken, NJ: Wiley, 2000
-
- Kyriacou DN, Lewis RJ.. Confounding by indication in clinical research. JAMA 2016; 316: 1818–1819 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

