An in-silico Investigation Into the Role of Strain and Structure on Vascular Smooth Muscle Cell Growth
- PMID: 33959595
- PMCID: PMC8093633
- DOI: 10.3389/fbioe.2021.641794
An in-silico Investigation Into the Role of Strain and Structure on Vascular Smooth Muscle Cell Growth
Abstract
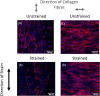
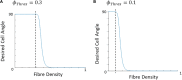
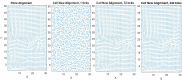
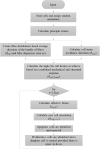
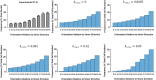
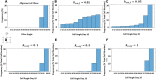
The orientation of vascular cells can greatly influence the in vivo mechanical properties and functionality of soft vascular tissues. How cell orientation mediates the growth response of cells is of critical importance in understanding the response of soft tissues to mechanical stimuli or injury. To date, considerable evidence has shown that cells align with structural cues such as collagen fibers. However, in the presence of uniaxial cyclic strain on unstructured substrates, cells generally align themselves perpendicularly to the mechanical stimulus, such as strain, a phenomenon known as "strain avoidance." The cellular response to this interplay between structural cues and a mechanical stimulus is poorly understood. A recent in vitro experimental study in our lab has investigated both the individual and collective response of rat aortic smooth muscle cells (RASMC) to structural (collagenous aligned constructs) and mechanical (cyclic strain) cues. In this study, a 2D agent-based model (ABM) is developed to simulate the collective response of RASMC to varying amplitudes of cyclic strain (0-10%, 2-8%, 4-6%) when seeded on unstructured (PDMS) and structured (decellularized collagenous tissue) constructs. An ABM is presented that is fit to the experimental outcomes in terms of cellular alignment and cell growth on PDMS substrates, under cyclic strain amplitudes of (4-6%, 2-8%, 0-10%) at 24 and 72 h timepoints. Furthermore, the ABM can predict RASMC alignment and change in cell number on a structured construct at a cyclic strain amplitude of 0-10% after 10 days. The ABM suggests that strain avoidance behavior observed in cells is dominated by selective cell proliferation and apoptosis at these early time points, as opposed to cell re-orientation, i.e., cells perpendicular to the strain increase their rate of proliferation, whilst the rate of apoptosis simultaneously increases in cells parallel to the strain direction. The development of in-silico modeling platforms, such as that presented here, allow for further understanding of the response of cells to changes in their mechanical environment. Such models offer an efficient and robust means to design and optimize the compliance and topological structure of implantable devices and could be used to aid the design of next-generation vascular grafts and stents.
Keywords: agent-based model; collagen; mechanobiology; reorientation; stretch-avoidance; vascular smooth muscle cells.
Copyright © 2021 McGee, Nolan, Mathieu and Lally.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Berens P. (2009). CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 31 1–21. 10.18637/jss.v031.i10 - DOI
-
- Colombo A. (2009). The Role of Altered Cyclic Strain Patterns on Proliferation and Apoptosis of Vascular Smooth Muscle Cells - Implications for in-Stent Restenosis. Ph. D. Thesis, Dublin City University, Dublin.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

