Isoalantolactone Increases the Sensitivity of Prostate Cancer Cells to Cisplatin Treatment by Inducing Oxidative Stress
- PMID: 33959604
- PMCID: PMC8093765
- DOI: 10.3389/fcell.2021.632779
Isoalantolactone Increases the Sensitivity of Prostate Cancer Cells to Cisplatin Treatment by Inducing Oxidative Stress
Abstract
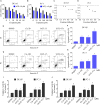
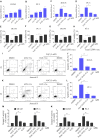
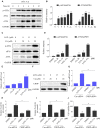
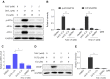
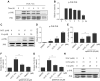
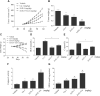
Prostate cancer is the most common malignancy among men worldwide. Platinum (II)-based chemotherapy has been used to treat a number of malignancies including prostate cancer. However, the potential of cisplatin for treating prostate cancer is restricted owing to its limited efficacy and toxic side effects. Combination therapies have been proposed to increase the efficacy and reduce the toxic side effects. In the present study, we investigated how isoalantolactone (IATL), a sesquiterpene lactone extracted from the medicinal plant Inula helenium L., acts synergistically with cisplatin on human prostate cancer cells. We show that IATL significantly increased cisplatin-induced growth suppression and apoptosis in human prostate cancer cells. Mechanistically, the combined treatment resulted in an excessive accumulation of intracellular reactive oxygen species (ROS), which leads to the activation of endoplasmic reticulum (ER) stress and the JNK signaling pathway in human prostate cancer cells. Pretreatment of cells with the ROS scavenger N-acetylcysteine (NAC) significantly abrogated the combined treatment-induced ROS accumulation and cell apoptosis. In addition, the activation of ER stress and the JNK signaling pathway prompted by IATL and cisplatin was also reversed by NAC pretreatment. In vivo, we found that IATL combined with cisplatin showed the strongest antitumor effects compared with single agents. These results support the notion that IATL and cisplatin combinational treatment may be more effective for treating prostate cancer than cisplatin alone.
Keywords: cisplatin; endoplasmic reticulum stress; isoalantolactone; oxidative stress; prostate cancer.
Copyright © 2021 Huang, Li, Ye, Zhang, Lin, Wu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

