Theileria's Strategies and Effector Mechanisms for Host Cell Transformation: From Invasion to Immortalization
- PMID: 33959614
- PMCID: PMC8096294
- DOI: 10.3389/fcell.2021.662805
Theileria's Strategies and Effector Mechanisms for Host Cell Transformation: From Invasion to Immortalization
Abstract
One of the first events that follows invasion of leukocytes by Theileria sporozoites is the destruction of the surrounding host cell membrane and the rapid association of the intracellular parasite with host microtubules. This is essential for the parasite to establish its niche within the cytoplasm of the invaded leukocyte and sets Theileria spp. apart from other members of the apicomplexan phylum such as Toxoplasma gondii and Plasmodium spp., which reside within the confines of a host-derived parasitophorous vacuole. After establishing infection, transforming Theileria species (T. annulata, T. parva) significantly rewire the signaling pathways of their bovine host cell, causing continual proliferation and resistance to ligand-induced apoptosis, and conferring invasive properties on the parasitized cell. Having transformed its target cell, Theileria hijacks the mitotic machinery to ensure its persistence in the cytoplasm of the dividing cell. Some of the parasite and bovine proteins involved in parasite-microtubule interactions have been fairly well characterized, and the schizont expresses at least two proteins on its membrane that contain conserved microtubule binding motifs. Theileria-encoded proteins have been shown to be translocated to the host cell cytoplasm and nucleus where they have the potential to directly modify signaling pathways and host gene expression. However, little is known about their mode of action, and even less about how these proteins are secreted by the parasite and trafficked to their target location. In this review we explore the strategies employed by Theileria to transform leukocytes, from sporozoite invasion until immortalization of the host cell has been established. We discuss the recent description of nuclear pore-like complexes that accumulate on membranes close to the schizont surface. Finally, we consider putative mechanisms of protein and nutrient exchange that might occur between the parasite and the host. We focus in particular on differences and similarities with recent discoveries in T. gondii and Plasmodium species.
Keywords: Apicomplexa; Plasmodium; Theileria; Toxoplasma; annulate lamellae; invasion; microtubule; transformation.
Copyright © 2021 Woods, Perry, Brühlmann and Olias.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a shared affiliation with the authors at the time of review.
Figures
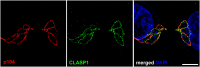

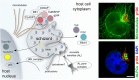
References
-
- Adamson R., Logan M., Kinnaird J., Langsley G., Hall R. (2000). Loss of matrix metalloproteinase 9 Activity in Theileria annulata-attenuated cells is at the transcriptional level and is associated with differentially expressed AP-1 species. Mol. Biochem. Parasitol. 106 51–61. 10.1016/s0166-6851(99)00213-3 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

