Shark Antibody Variable Domains Rigidify Upon Affinity Maturation-Understanding the Potential of Shark Immunoglobulins as Therapeutics
- PMID: 33959632
- PMCID: PMC8093575
- DOI: 10.3389/fmolb.2021.639166
Shark Antibody Variable Domains Rigidify Upon Affinity Maturation-Understanding the Potential of Shark Immunoglobulins as Therapeutics
Abstract
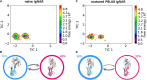
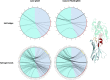
Sharks and other cartilaginous fish are the phylogenetically oldest living organisms that have antibodies as part of their adaptive immune system. As part of their humoral adaptive immune response, they produce an immunoglobulin, the so-called immunoglobulin new antigen receptor (IgNAR), a heavy-chain only antibody. The variable domain of an IgNAR, also known as V NAR , binds the antigen as an independent soluble domain. In this study, we structurally and dynamically characterized the affinity maturation mechanism of the germline and somatically matured (PBLA8) V NAR to better understand their function and their applicability as therapeutics. We observed a substantial rigidification upon affinity maturation, which is accompanied by a higher number of contacts, thereby contributing to the decrease in flexibility. Considering the static x-ray structures, the observed rigidification is not obvious, as especially the mutated residues undergo conformational changes during the simulation, resulting in an even stronger network of stabilizing interactions. Additionally, the simulations of the V NAR in complex with the hen egg-white lysozyme show that the V NAR antibodies evidently follow the concept of conformational selection, as the binding-competent state already preexisted even without the presence of the antigen. To have a more detailed description of antibody-antigen recognition, we also present here the binding/unbinding mechanism between the hen egg-white lysozyme and both the germline and matured V NAR s. Upon maturation, we observed a substantial increase in the resulting dissociation-free energy barrier. Furthermore, we were able to kinetically and thermodynamically describe the binding process and did not only identify a two-step binding mechanism, but we also found a strong population shift upon affinity maturation toward the native binding pose.
Keywords: VNAR; affinity maturation; binding interfaces; binding mechanisms; conformational selection; encounter complex; shark antibodies.
Copyright © 2021 Fernández-Quintero, Seidler, Quoika and Liedl.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
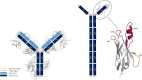
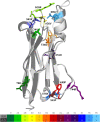


References
-
- Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C., Hess B., et al. (2015). GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1– 2 19–25. 10.1016/j.softx.2015.06.001 - DOI
-
- Adelman S. A., Doll J. D. (1976). Generalized Langevin equation approach for atom/solid−surface scattering: general formulation for classical scattering off harmonic solids. J. Chem. Phys. 64 2375–2388. 10.1063/1.432526 - DOI
-
- Alessandro L., Gervasio F. L. (2008). Metadynamics: a method to simulate rare events and reconstruct the free energy in biophysics, chemistry and material science. Rep. Progr. Phys. 71:126601. 10.1088/0034-4885/71/12/126601 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

