Impact of Ribosome Activity on SARS-CoV-2 LNP - Based mRNA Vaccines
- PMID: 33959636
- PMCID: PMC8093617
- DOI: 10.3389/fmolb.2021.654866
Impact of Ribosome Activity on SARS-CoV-2 LNP - Based mRNA Vaccines
Abstract
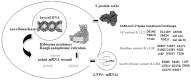
Coronavirus-related Severe Acute Respiratory Syndrome-2 (SARS-CoV-2) initially was detected in Wuhan, Hubei, China. Since early 2021, World Health Organization (WHO) has declared Coronavirus Disease 2019 (COVID-19) a pandemic due to rapidly transformed to a globally massive catastrophic viral infection. In order to confront this emergency situation, many pharmaceutical companies focused on the design and development of efficient vaccines that are considered necessary for providing a level of normalization in totally affected human social-economical activity worldwide. A variety of vaccine types are under development, validation or even some of them have already completed these stages, initially approved as conditional marketing authorisation by Food and Drug Administration (FDA), European Medicines Agency (EMA), and other national health authorities for commercial purposes (in vivo use in general population), accelerating their production and distribution process. Innovative nucleoside-modified viral messenger RNA (v-mRNA)-based vaccines encapsulated within nanoparticles-specifically lipid ones (LNPs)-are now well recognized. Although this is a promising genetic engineering topic in the field of nanopharmacogenomics or targeted nucleic vaccines, there are limited but continuously enriched in vivo data in depth of time regarding their safety, efficacy, and immune response. In the current paper we expand the limited published data in the field of ribosome machinery and SARS-CoV-2 mRNA fragment vaccines interaction by describing their functional specialization and modifications. Additionally, alterations in post-transcriptional/translational molecules and mechanisms that could potentially affect the interaction between target cells and vaccines are also presented. Understanding these mechanisms is a crucial step for the next generation v-mRNA vaccines development.
Keywords: COVID-19; LNP; SARS-CoV-2; mRNA; ribosome; vaccine.
Copyright © 2021 Tsiambas, Chrysovergis, Papanikolaou, Mastronikolis, Ragos, Batistatou, Peschos, Kavantzas, Lazaris and Kyrodimos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

