Efficacy and safety of subcutaneous prophylaxis with dalcinonacog alfa in adults with haemophilia B
- PMID: 33960073
- PMCID: PMC8359950
- DOI: 10.1111/hae.14315
Efficacy and safety of subcutaneous prophylaxis with dalcinonacog alfa in adults with haemophilia B
Abstract
Aim: Phase 2b study to assess efficacy, safety, thrombogenicity, immunogenicity and tolerability with 28 days of daily dosing of subcutaneous (SQ) dalcinonacog alfa as prophylaxis for haemophilia B (HB).
Methods: Adult males with a confirmed diagnosis of congenital HB (factor IX [FIX] activity <2%) received daily dalcinonacog alfa 100 IU/kg SQ until day 28. The primary efficacy endpoint was the number of participants who achieved a steady-state FIX activity level ≥12%. Tolerability, thrombogenicity and immunogenicity were study safety endpoints.
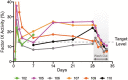
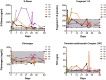
Results: Of 6 participants who received study drug, one discontinued the study on day 7 due to injection-site reactions (ISR). Of the 5 participants completing the study, FIX activity level exceeded 12% in 3 participants at day 7, increasing to 4 participants on days 14, 21 and 28 and all 5 at day 29. Pharmacokinetic findings (including mean alpha and beta half-life of 5.3 days and 3.9 days, respectively, and mean residence time of 6.2 days) supported prolonged effects. Thrombogenicity markers remained normal throughout prophylactic injections or showed some initial increases followed by decreases with continued dosing. Two participants had anti-drug antibodies to dalcinonacog alfa at study end, none had neutralizing antibody. Two participants had ISR, both resolved. Reports of redness, swelling, tenderness or pain among the first 3 participants prompted dose-splitting for the last 3 participants, leading to fewer ISR.
Conclusion: Subcutaneous dalcinonacog alfa is effective in raising FIX levels into the mild haemophilia range, comparable to intravenous extended half-life FIX clotting factors.
Keywords: clinical trial; dalcinonacog alfa; factor IX; haemophilia B; immunogenicity; prophylaxis; subcutaneous.
© 2021 Catalyst Biosciences. Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
JM has received research grants from Bayer, Biogen, Biomarin, CSL, Novo Nordisk, Sobi, Roche and Unique; has served as a member of the scientific advisory committee of Amgen, Bayer, Biotest, Biogen, Baxalta, CSL Behring, Catalyst Biosciences, Novo Nordisk, Roche and Spark; and has served as a member of the speaker bureau of Alnylam, Bayer, Biotest, Biogen, Novo Nordisk, Pfizer, Sobi, Shire, Roche, ISTH and WFH. HL and FDG are employees and stockholders of Catalyst Biosciences. ML has acted as a paid consultant to Catalyst Biosciences.
Figures
References
-
- Mannucci PM, Tuddenham EG. The hemophilias−from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773‐1779. - PubMed
-
- Giangrande P. Haemophilia B: Christmas disease. Expert Opin Pharmacother. 2005;6(9):1517‐1524. - PubMed
-
- Fischer K, Van Den Berg M. Prophylaxis for severe haemophilia: clinical and economical issues. Haemophilia. 2003;9(4):376‐381. - PubMed
-
- Ljung RC. Prophylactic infusion regimens in the management of hemophilia. Thromb Haemost. 1999;82(2):525‐530. - PubMed
-
- Manco‐Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535‐544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical