Optimization, and in vitro and in vivo evaluation of etomidate intravenous lipid emulsion
- PMID: 33960250
- PMCID: PMC8118403
- DOI: 10.1080/10717544.2021.1917729
Optimization, and in vitro and in vivo evaluation of etomidate intravenous lipid emulsion
Abstract
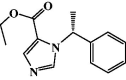
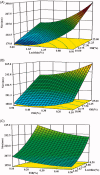
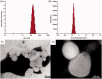
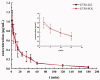
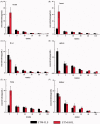
The aim of this investigation was to develop an etomidate intravenous lipid emulsion (ETM-ILE) and evaluate its properties in vitro and in vivo. Etomidate (ETM) is a hydrophobic drug, and organic solvents must be added to an etomidate injectable solution (ETM-SOL) to aid dissolution, that causes various adverse reactions on injection. Lipid emulsions are a novel drug formulation that can improve drug loading and reduce adverse reactions. ETM-ILE was prepared using high-pressure homogenization. Univariate experiments were performed to select key conditions and variables. The proportion of oil, egg lecithin, and poloxamer 188 (F68) served as variables for the optimization of the ETM-ILE formulation by central composite design response surface methodology. The optimized formulation had the following characteristics: particle size, 168.0 ± 0.3 nm; polydispersity index, 0.108 ± 0.028; zeta potential, -36.4 ± 0.2 mV; drug loading, 2.00 ± 0.01 mg/mL; encapsulation efficiency, 97.65% ± 0.16%; osmotic pressure, 292 ± 2 mOsmol/kg and pH value, 7.63 ± 0.07. Transmission electron microscopy images showed that the particles were spherical or spheroidal, with a diameter of approximately 200 nm. The stability study suggested that ETM-ILE could store at 4 ± 2 °C or 25 ± 2 °C for 12 months. Safety tests showed that ETM-ILE did not cause hemolysis or serious vascular irritation. The results of the pharmacokinetic study found that ETM-ILE was bioequivalent to ETM-SOL. However, a higher concentration of ETM was attained in the liver, spleen, and lungs after administration of ETM-ILE than after administration of ETM-SOL. This study found that ETM-ILE had great potential for clinical applications.
Keywords: Etomidate; intravenous lipid emulsion; pharmacokinetics; response surface methodology; tissue distribution.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Development of ligustrazine-loaded lipid emulsion: formulation optimization, characterization and biodistribution.Int J Pharm. 2012 Nov 1;437(1-2):203-12. doi: 10.1016/j.ijpharm.2012.08.027. Epub 2012 Aug 28. Int J Pharm. 2012. PMID: 22944305
-
Lipid emulsion as a drug delivery system for breviscapine: formulation development and optimization.Arch Pharm Res. 2012 Jun;35(6):1037-43. doi: 10.1007/s12272-012-0611-z. Epub 2012 Jun 30. Arch Pharm Res. 2012. PMID: 22870813
-
Preparation and in vivo evaluation of an intravenous emulsion loaded with an aprepitant-phospholipid complex.Drug Deliv. 2023 Dec;30(1):2183834. doi: 10.1080/10717544.2023.2183834. Drug Deliv. 2023. PMID: 36843571 Free PMC article.
-
Novel etomidate derivatives.Curr Pharm Des. 2012;18(38):6253-6. doi: 10.2174/138161212803832362. Curr Pharm Des. 2012. PMID: 22762475 Review.
-
Etomidate and other non-barbiturates.Handb Exp Pharmacol. 2008;(182):267-82. doi: 10.1007/978-3-540-74806-9_13. Handb Exp Pharmacol. 2008. PMID: 18175096 Review.
Cited by
-
Emerging Anesthetic Nanomedicines: Current State and Challenges.Int J Nanomedicine. 2023 Jul 19;18:3913-3935. doi: 10.2147/IJN.S417855. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37489141 Free PMC article. Review.
-
Solubilization techniques used for poorly water-soluble drugs.Acta Pharm Sin B. 2024 Nov;14(11):4683-4716. doi: 10.1016/j.apsb.2024.08.027. Epub 2024 Sep 2. Acta Pharm Sin B. 2024. PMID: 39664427 Free PMC article. Review.
-
Nanotherapeutics for Alleviating Anesthesia-Associated Complications.Adv Sci (Weinh). 2024 Apr;11(15):e2308241. doi: 10.1002/advs.202308241. Epub 2024 Feb 11. Adv Sci (Weinh). 2024. PMID: 38342603 Free PMC article. Review.
-
In Vitro and In Vivo Evaluation of Lactoferrin-Modified Liposomal Etomidate with Enhanced Brain-Targeting Effect for General Anesthesia.Pharmaceutics. 2024 Jun 14;16(6):805. doi: 10.3390/pharmaceutics16060805. Pharmaceutics. 2024. PMID: 38931926 Free PMC article.
-
Preparation and Evaluation of Curcumin Derivatives Nanoemulsion Based on Turmeric Extract and Its Antidepressant Effect.Int J Nanomedicine. 2023 Dec 27;18:7965-7983. doi: 10.2147/IJN.S430769. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38162571 Free PMC article.
References
-
- Agrawal U, Chashoo G, Sharma PR, et al. (2015). Tailored polymer-lipid hybrid nanoparticles for the delivery of drug conjugate: dual strategy for brain targeting. Colloids Surf B Biointerfaces 126:414–25. - PubMed
-
- Carpentier YA, Simoens C, Siderova V, et al. (1997). Recent developments in lipid emulsions: relevance to intensive care. Nutrition 13:73s–8s. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
