Amine-functionalized carbon-fiber microelectrodes for enhanced ATP detection with fast-scan cyclic voltammetry
- PMID: 33960336
- PMCID: PMC8202729
- DOI: 10.1039/d1ay00089f
Amine-functionalized carbon-fiber microelectrodes for enhanced ATP detection with fast-scan cyclic voltammetry
Abstract
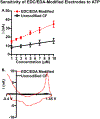
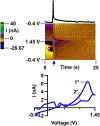
Here, we provide evidence that functionalizing the carbon-fiber surface with amines significantly improves direct electrochemical adenosine triphosphate (ATP) detection with fast-scan cyclic voltammetry (FSCV). ATP is an important extracellular signaling molecule throughout the body and can function as a neurotransmitter in the brain. Several methods have been developed over the years to monitor and quantitate ATP signaling in cells and tissues; however, many of them are limited in temporal resolution or are not capable of measuring ATP directly. FSCV at carbon-fiber microelectrodes is a widely used technique to measure neurotransmitters in real-time. Many electrode treatments have been developed to study the interaction of cationic compounds like dopamine at the carbon surface yet studies investigating how to improve anionic compounds, like ATP, at the carbon fiber surface are lacking. In this work, carbon-fibers were treated with N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide (EDC) which reacts with carboxylic acid groups on the carbon surface followed by reaction with ethylenediamine (EDA) to produce NH2-functionalized carbon surfaces. Overall, we a 5.2 ± 2.5-fold increase in ATP current with an approximately 9-fold increase in amine functionality, as analyzed by X-ray Photoelectron Spectroscopy, on the carbon surface was observed after modification with EDC-EDA. This provides evidence that amine-rich surfaces improve interactions with ATP on the surface. This study provides a detailed analysis of ATP interaction at carbon surfaces and ultimately a method to improve direct and rapid neurological ATP detection in the future.
Figures
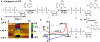


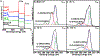


Similar articles
-
Plasma-treated carbon-fiber microelectrodes for improved purine detection with fast-scan cyclic voltammetry.Analyst. 2020 Feb 3;145(3):805-815. doi: 10.1039/c9an01636h. Analyst. 2020. PMID: 31820742
-
Metal Nanoparticle Modified Carbon-Fiber Microelectrodes Enhance Adenosine Triphosphate Surface Interactions with Fast-Scan Cyclic Voltammetry.ACS Meas Sci Au. 2022 Apr 20;2(2):96-105. doi: 10.1021/acsmeasuresciau.1c00026. Epub 2021 Oct 7. ACS Meas Sci Au. 2022. PMID: 35479102 Free PMC article.
-
Plasma-treated gold microelectrodes for subsecond detection of Zn(II) with fast-scan cyclic voltammetry.Analyst. 2024 Sep 9;149(18):4643-4652. doi: 10.1039/d4an00307a. Analyst. 2024. PMID: 39136087
-
Fast-scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release.Comput Struct Biotechnol J. 2014 Dec 29;13:47-54. doi: 10.1016/j.csbj.2014.12.006. eCollection 2015. Comput Struct Biotechnol J. 2014. PMID: 26900429 Free PMC article. Review.
-
Recent advances in fast-scan cyclic voltammetry.Analyst. 2020 Feb 17;145(4):1087-1102. doi: 10.1039/c9an01925a. Analyst. 2020. PMID: 31922162 Free PMC article. Review.
Cited by
-
Platinum Nanoparticle Size and Density Impacts Purine Electrochemistry with Fast-Scan Cyclic Voltammetry.J Electrochem Soc. 2022 Apr;169(4):046514. doi: 10.1149/1945-7111/ac65bc. Epub 2022 Apr 19. J Electrochem Soc. 2022. PMID: 35497383 Free PMC article.
-
Dioxythiophene/Nafion Polymer Composite Membranes for Tunable Size-Based Selectivity in the Voltammetric Detection of Small Neuropeptides.ACS Sens. 2024 Oct 25;9(10):5109-5115. doi: 10.1021/acssensors.4c00848. Epub 2024 Sep 25. ACS Sens. 2024. PMID: 39319559
-
Electrodeposition of dopamine onto carbon fiber microelectrodes to enhance the detection of Cu2+ via fast-scan cyclic voltammetry.Anal Bioanal Chem. 2023 Jul;415(18):4289-4296. doi: 10.1007/s00216-022-04488-4. Epub 2023 Jan 3. Anal Bioanal Chem. 2023. PMID: 36595035
-
Nanostructured carbon-fiber surfaces for improved neurochemical detection.Faraday Discuss. 2022 Apr 5;233(0):336-353. doi: 10.1039/d1fd00049g. Faraday Discuss. 2022. PMID: 34935021 Free PMC article.
-
Editors' Choice-Review-The Future of Carbon-Based Neurochemical Sensing: A Critical Perspective.ECS Sens Plus. 2023 Dec 1;2(4):043601. doi: 10.1149/2754-2726/ad15a2. Epub 2023 Dec 27. ECS Sens Plus. 2023. PMID: 38170109 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

