Comparison of the effects of two therapeutic strategies based on olfactory ensheathing cell transplantation and repetitive magnetic stimulation after spinal cord injury in female mice
- PMID: 33960512
- PMCID: PMC8359979
- DOI: 10.1002/jnr.24836
Comparison of the effects of two therapeutic strategies based on olfactory ensheathing cell transplantation and repetitive magnetic stimulation after spinal cord injury in female mice
Abstract
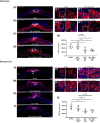
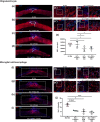
Spinal cord injury (SCI) is a debilitating condition, which leads to a permanent loss of functions below the injury site. The events which take place after SCI are characterized by cellular death, release of inhibitory factors, and inflammation. Many therapies have been studied to cure SCI, among them magnetic stimulation aims to reduce the secondary damages in particular by decreasing apoptosis, while, cellular transplantation promotes neuroregeneration by enhancing axonal regrowth. In the present study, we compared individually primary olfactory ensheathing cell (OEC) transplantation and repetitive trans-spinal magnetic stimulation (rTSMS) and then, we combined these two therapeutic approaches on tissue repair and functional recovery after SCI. To do so, SCIs were performed at Th10 level on female C57BL/6 mice, which were randomized into four groups: SCI, SCI + primary bOECs, SCI + STM, SCI + primary bulbar olfactory ensheathing cells (bOECs) + stimulation (STM). On these animals bioluminescence, immunohistological, and behavioral experiments were performed after SCI. Our results show that rTSMS has beneficial effect on the modulation of spinal scar by reducing fibrosis, demyelination, and microglial cell activation and by increasing the astroglial component of the scar, while, primary bOEC transplantation decreases microglial reactivity. At the opposite, locotronic experiments show that both treatments induce functional recovery. We did not observed any additional effect by combining the two therapeutic approaches. Taken together, the present study indicates that primary bOEC transplantation and rTSMS treatment act through different mechanisms after SCI to induce functional recovery. In our experimental paradigm, the combination of the two therapies does not induce any additional benefit.
Keywords: RRID:AB_10563302: PDGFRβ, Abcam, ab91066; RRID:AB_10643424: PE, poly4064, BioLegend, 406408; RRID:AB_2313568: Jackson ImmunoResearch, 711-166-152; RRID:AB_2340667: Jackson ImmunoResearch, 712-165-153; RRID:AB_2340812: Jackson ImmunoResearch, 715-165-140; RRID:AB_2715913: Alexa 488, MRG2b-85, BioLegend; RRID:AB_306827: p75, Abcam, ab8874; RRID:AB_476889: GFAP Cy3-conjugated Sigma-Aldrich, C9205; RRID:AB_777165:P DGFRβAbcam ab32570; RRID:AB_839504: Iba1, Wako, 019-19741; RRID:AB_94975: MBP, Millipore, MAB386; RRID:IMSR_JAX:008450: L2G85Chco+/+ (FVB-Tg(CAG-luc,-GFP)L2G85Chco/J); glial scar; magnetic stimulation; olfactory ensheathing cells and neuroregeneration; rehabilitation; spinal cord injury.
© 2021 The Authors. Journal of Neuroscience Research published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Inhibition of ADAMTS-4 Expression in Olfactory Ensheathing Cells Enhances Recovery after Transplantation within Spinal Cord Injury.J Neurotrauma. 2020 Feb 1;37(3):507-516. doi: 10.1089/neu.2019.6481. Epub 2019 Aug 27. J Neurotrauma. 2020. PMID: 31264504
-
The Regenerative Effect of Trans-spinal Magnetic Stimulation After Spinal Cord Injury: Mechanisms and Pathways Underlying the Effect.Neurotherapeutics. 2020 Oct;17(4):2069-2088. doi: 10.1007/s13311-020-00915-5. Neurotherapeutics. 2020. PMID: 32856173 Free PMC article.
-
Conditioned medium of olfactory ensheathing cells promotes the functional recovery and axonal regeneration after contusive spinal cord injury.Brain Res. 2017 Jan 1;1654(Pt A):43-54. doi: 10.1016/j.brainres.2016.10.023. Epub 2016 Oct 24. Brain Res. 2017. PMID: 27789279
-
[Pathogenesis of spinal cord injuries and mechanisms of repair induced by olfactory ensheathing cells].Rev Neurol. 2013 May 16;56(10):521-31. Rev Neurol. 2013. PMID: 23658035 Review. Spanish.
-
Optimizing Olfactory Ensheathing Cell Transplantation for Spinal Cord Injury Repair.J Neurotrauma. 2020 Mar 1;37(5):817-829. doi: 10.1089/neu.2019.6939. J Neurotrauma. 2020. PMID: 32056492 Review.
Cited by
-
The therapeutic mechanism of transcranial iTBS on nerve regeneration and functional recovery in rats with complete spinal cord transection.Front Immunol. 2023 Jun 14;14:1153516. doi: 10.3389/fimmu.2023.1153516. eCollection 2023. Front Immunol. 2023. PMID: 37388732 Free PMC article.
-
Advances in Spinal Cord Neuromodulation: The Integration of Neuroengineering, Computational Approaches, and Innovative Conceptual Frameworks.J Pers Med. 2023 Jun 13;13(6):993. doi: 10.3390/jpm13060993. J Pers Med. 2023. PMID: 37373982 Free PMC article.
-
Repetitive Trans Spinal Magnetic Stimulation Improves Functional Recovery and Tissue Repair in Contusive and Penetrating Spinal Cord Injury Models in Rats.Biomedicines. 2021 Dec 3;9(12):1827. doi: 10.3390/biomedicines9121827. Biomedicines. 2021. PMID: 34944643 Free PMC article.
-
Designing a Clinical Trial with Olfactory Ensheathing Cell Transplantation-Based Therapy for Spinal Cord Injury: A Position Paper.Biomedicines. 2022 Dec 6;10(12):3153. doi: 10.3390/biomedicines10123153. Biomedicines. 2022. PMID: 36551909 Free PMC article. Review.
-
Contribution of glial cells to the neuroprotective effects triggered by repetitive magnetic stimulation: a systematic review.Neural Regen Res. 2024 Jan;19(1):116-123. doi: 10.4103/1673-5374.374140. Neural Regen Res. 2024. PMID: 37488852 Free PMC article. Review.
References
-
- Ahuja, C. S., Mothe, A., Khazaei, M., Badhiwala, J. H., Gilbert, E. A., van der Kooy, D., Morshead, C. M., Tator, C., & Fehlings, M. G. (2020). The leading edge: Emerging neuroprotective and neuroregenerative cell‐based therapies for spinal cord injury. Stem Cells Translational Medicine, 9, 1509–1530. 10.1002/sctm.19-0135 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

