Functional connectivity of the human hypothalamus during wakefulness and nonrapid eye movement sleep
- PMID: 33960583
- PMCID: PMC8249893
- DOI: 10.1002/hbm.25461
Functional connectivity of the human hypothalamus during wakefulness and nonrapid eye movement sleep
Abstract
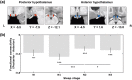
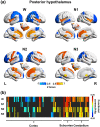
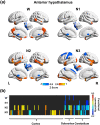
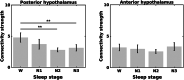
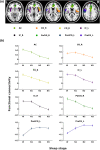
Animal experiments indicate that the hypothalamus plays an essential role in regulating the sleep-wake cycle. A recent neuroimaging study conducted under resting wakefulness conditions suggested the presence of a wake-promoting region and a sleep-promoting region in the human posterior hypothalamus and anterior hypothalamus, respectively, and interpreted their anticorrelated organization in resting-state functional networks as evidence for their opposing roles in sleep-wake regulation. However, whether and how the functional networks of the two hypothalamic regions reorganize according to their wake- or sleep-promoting roles during sleep are unclear. Here, we constructed functional networks of the posterior and anterior hypothalamus during wakefulness and nonrapid eye movement (NREM) sleep using simultaneous electroencephalography and functional magnetic resonance imaging data collected from 62 healthy participants. The functional networks of the posterior and anterior hypothalamus exhibited inversely correlated organizations during both wakefulness and NREM sleep. The connectivity strength of the posterior hypothalamic functional network was stronger during wakefulness than during stable sleep. From wakefulness to sleep, the anterior cingulate gyrus, paracingulate gyrus, insular cortex, and fontal operculum cortex showed decreased positive connectivity, while the precentral gyrus and postcentral gyrus showed decreased negative connectivity with the posterior hypothalamus. Additionally, the insular cortex and frontal operculum cortex showed negative connectivity during wakefulness and positive connectivity during sleep with the anterior hypothalamus, exhibiting an increasing trend. These findings provide insights into the correspondence between the functional network organizations and hypothalamic sleep-wake regulation in humans.
Keywords: functional connectivity; hypothalamus; sleep-wake regulation; tuberomammillary nucleus; ventrolateral preoptic nucleus.
© 2021 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Allen, P. J. , Josephs, O. , & Turner, R. (2000). A method for removing imaging artifact from continuous EEG recorded during functional MRI. NeuroImage, 12, 230–239. - PubMed
-
- Allen, P. J. , Polizzi, G. , Krakow, K. , Fish, D. R. , & Lemieux, L. (1998). Identification of EEG events in the MR scanner: The problem of pulse artifact and a method for its subtraction. NeuroImage, 8, 229–239. - PubMed
-
- Baroncini, M. , Jissendi, P. , Balland, E. , Besson, P. , Pruvo, J. P. , Francke, J. P. , … Prevot, V. (2012). MRI atlas of the human hypothalamus. NeuroImage, 59, 168–180. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

