Utility of red-light ultrafast optogenetic stimulation of the auditory pathway
- PMID: 33960685
- PMCID: PMC8185542
- DOI: 10.15252/emmm.202013391
Utility of red-light ultrafast optogenetic stimulation of the auditory pathway
Abstract
Optogenetic stimulation of spiral ganglion neurons (SGNs) in the ear provides a future alternative to electrical stimulation used in current cochlear implants. Here, we employed fast and very fast variants of the red-light-activated channelrhodopsin (ChR) Chrimson (f-Chrimson and vf-Chrimson) to study their utility for optogenetic stimulation of SGNs in mice. The light requirements were higher for vf-Chrimson than for f-Chrimson, even when optimizing membrane expression of vf-Chrimson by adding potassium channel trafficking sequences. Optogenetic time and intensity coding by single putative SGNs were compared with coding of acoustic clicks. vf-Chrimson enabled putative SGNs to fire at near-physiological rates with good temporal precision up to 250 Hz of stimulation. The dynamic range of SGN spike rate coding upon optogenetic stimulation was narrower than for acoustic clicks but larger than reported for electrical stimulation. The dynamic range of spike timing, on the other hand, was more comparable for optogenetic and acoustic stimulation. In conclusion, f-Chrimson and vf-Chrimson are promising candidates for optogenetic stimulation of SGNs in auditory research and future cochlear implants.
Keywords: channelrhodopsin; cochlear implant; dynamic range; gating; spiral ganglion; temporal coding.
© 2021 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
TM is a co‐founder and CEO of OptoGenTech company. The other authors declare no conflict of interests.
Figures
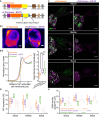
pAAV vector used in the study containing vf‐Chrimson‐eYFP (“vf‐Chrimson”, upper) or with a trafficking signal (TS), eYFP and ER export signal (ES) vf‐Chrimson‐eYFP (“vf‐Chrimson‐ES/TS”, lower). In each, expression was driven by the human synapsin promoter (hSyn) and enhanced by the Woodchuck hepatitis virus posttranslational regulatory element (WPRE) and bovine growth hormone (bGH) polyadenylation signal (bGH poly A) sequences. ITR: inverted terminal repeats.
(Bi) Confocal images of eYFP‐immunolabeled SGN somata transduced by either construct: Warmer colors represent higher fluorescence intensity indicating subcellular distribution of the channelrhodopsin expression. Enhanced localization of the opsin to the plasma membrane (arrowheads) is obvious when employing the ES/TS‐trafficking signals. Scale bars: 2 µm. (Bii) Quantification of membrane localization of vf‐Chrimson where fluorescence intensity of the immunofluorescence of the anti‐YFP‐antibody (mean ± SEM, line ± shaded area) is plotted across the cell membrane (n = 30 cells, N = 5 mice per group). The position of the plasma membrane was approximated where parvalbumin (PV) immunofluorescence of SGNs reached 50% (dashed line). For comparison, a f‐Chrimson line profile was analyzed based on Mager et al (2018). Right panel: Box‐and‐whisker plots (minimum, 25th, median, 75th percentile, and maximum) of ratio of maximum membrane fluorescence to maximum intracellular fluorescence (n = 30 cells, N = 5 mice per group). vf‐Chrimson scored around 1, which is lower compared to vf‐Chrimson‐ES/TS and f‐Chrimson (f‐Chrimson has intrinsically high membrane expression; P‐value = 1.1 × 10−8, Kruskal–Wallis test followed by Tukey's multiple comparison test).
Representative maximum‐projection confocal images of fluorescently labeled mid‐modiolar cryosections of injected cochleae for vf‐Chrimson and vf‐Chrimson‐ES/TS: PV‐positive SGNs (magenta) and transduced SGNs (green) in apex, mid, and base of the cochlea. Scale bars= 50 µm.
Box‐and‐whisker plots (minimum, 25th, median, 75th percentile, and maximum) of the percentage of YFP‐positive SGNs for all turns of injected (dark color) or contralateral non‐injected (light color) cochleae. The horizontal line within the box indicates the median, boundaries of the box indicate the 0.25‐ and 0.75‐percentile, and the whiskers indicate the highest and lowest values of the results. No significant differences are observed between vf‐Chrimson and vf‐Chrimson‐ES/TS‐injected ears (N = 15 mice for vf‐Chrimson; N = 9 mice for vf‐Chrimson‐ES/TS; P = 0.3596, Mann–Whitney U‐test).
Box‐and‐whisker plots (minimum, 25th, median, 75th percentile, and maximum) of the SGN density (number of PV‐positive SGN somata per cross‐sectional area of Rosenthal’s canal) for all turns of injected (dark color) or contralateral non‐injected (light color) cochleae. No significant differences were observed between vf‐Chrimson and vf‐Chrimson‐ES/TS in the injected ear (P‐value = 0.1060, Mann–Whitney test) nor between injected and non‐injected cochleae of either construct (vf‐Chrimson, N = 15 mice, P‐value = 0.2157; vf‐Chrimson‐ES/TS, N = 9 mice, P‐value = 0.6517, Mann–Whitney U‐test).

- A–F
Immunofluorescence of f‐Chrimson‐eYFP (A, C, E) and vf‐Chrimson‐eYFP (B, D, F) plotted across the plasma membrane and the membrane proximal intracellular regions. The line profiles are obtained from single‐stack confocal images of NG cells transfected with either construct (n = 30 cells, N = 4 transfections per construct). Line profiles are grouped according to the cellular distribution of the fluorescence. (A + B): intracellular fluorescence weaker than plasma membrane fluorescence, (C + D): intracellular fluorescence similar to plasma membrane fluorescence (E + F): gradual increase of fluorescence from plasma membrane to cell interior.
- G
Exemplary live cells, single‐stack confocal image of NG cells expressing f‐Chrimson‐eYFP. Scale bar = 10 µm.
- H
Bar graph summarizing the line profiles, grouped according to the cellular distribution of the fluorescence shown in (A‐F).
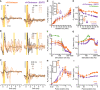
- A
Exemplary oABRs driven with varying radiant flux (1000 × 1 ms at 10 Hz, colors code the radiant flux in mW) of mice injected with AAV‐PHP.B‐vf‐Chrimson (left) or AAV‐PHP.B‐vf‐Chrimson‐ES/TS (right). First positive (P1) and first negative (N1) peaks are indicated by black dots on the waveform triggered upon 41.7 mW radiant flux (the initial negative deflection results from filtering). Vertical scale bar: 1 µV.
- B
Exemplary oABRs driven with varying stimulation rate (1 ms for data points ≤ 500 Hz and 0.5 ms above 500 Hz at 41.7 mW (highest radiant flux), colors code the stimulation rate) of the same animals from (A). Stimuli applied at a rate of 200 Hz and the corresponding P1‐N1 pairs are indicated by yellow shaded area and black dots, respectively. Vertical scale bar: 1 µV.
- C
oABRs driven with varying light pulse duration (10 Hz at 41.7 mW (highest radiant flux)), colors code the pulse duration) of the same animals from (A). Exemplary pulse duration of 1 ms and corresponding P1‐N1 pair are indicated by yellow shaded area and black dots. Vertical scale bar: 1 µV.
- D–I
Quantification of P1‐N1 amplitudes (mean ± SEM) and P1 latencies (mean ± SEM) as a function of radiant flux (D, E, vf‐Chrimson: N = 13 mice, vf‐Chrimson‐ES/TS: N = 9 mice), stimulation rate (F, G, vf‐Chrimson: N = 13 mice, vf‐Chrimson‐ES/TS: N = 8 mice), and pulse duration (H, I, vf‐Chrimson: N = 10 mice, vf‐Chrimson‐ES/TS: N = 9 mice). The average P1‐N1 amplitude of f‐Chrimson (green in F and G) is replotted from Mager et al, 2018. Inset in (D), quantification of the oABR threshold. Inset in (E), quantification of the shortest P1 latencies elicited among any radiant flux (****, P‐value < 10−4, Mann–Whitney U‐test). Inset in (H), quantification of the optimal pulse duration required to elicit the maximum P1‐N1 amplitude. Boxes show 25th percentile, median, 75th percentile, the black dot the mean, and whiskers maximum and minimum.
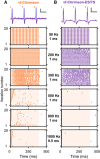
- A, B
Exemplary spike traces at 50 Hz, 1 ms, maximum radiant flux and corresponding raster plots at varying stimulation rates of vf‐Chrimson‐ (A, orange) and vf‐Chrimson‐ES/TS‐ (B, purple) expressing putative SGNs. Raster plots showing spiking activity of the above units in response to 400 ms‐long trains of laser pulses (shaded areas, at 43 mW, pulse duration = 1 ms between 20 and 800 Hz, pulse duration = 500 µs for ≥ 900 Hz) recorded at six different stimulation rates over 20 iterations. Scale bars 0.1 mV, 10 ms (A) and 1 mV, 10 ms (B).
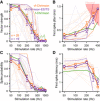
- A–D
Quantification of the vector strength (A), first spike jitter (B), spike probability (C), and first spike latency (D) as a function of the repetition rate of putative SGNs expressing vf‐Chrimson (orange, n = 28 putative SGNs) or vf‐Chrimson‐ES/TS (purple, n = 19 putative SGNs). Single SGNs are represented in light and mean ± SEM per vf‐Chrimson variant in color. f‐Chrimson data (green) were replotted from Mager et al (2018). The putative SGNs represented on Fig 3 are shown with dashed lines. In (B), the red‐shaded area represents the hazard function (i.e., the averaged first spike latency jitter measured from simulated Poisson spike train not containing any synchronization).

Raster plots from a vf‐Chrimson‐expressing putative SGN at a subset (0, 13, 17 20, 24 29 mW) of the different tested radiant fluxes.
Discharge rate as a function from the radiant flux from the SGN presented in (A).
Rate‐level function (RLF) of the SGN presented in (A). The laser power was calculated as follows: Laser power (in dB [mW]) = 10 × where A is the radiant flux (in mW) and A0 is the oABR threshold (in mW). The RLF was fitted using a sigmoidal fit (black line, R 2 = 0.99) and the dynamic range, displayed by a double arrow, defined as the level difference yielding a driven rate change equal to 90% of the maximum driving rate (Ohlemiller et al, 1991). The threshold was determined as the lowest laser power for which a d’ of 1, using the dark condition as reference and was computed from the discharge rate (Macmillan & Creelman, 2004; Huet et al, 2018).
First spike latency (FSL, square) and first spike jitter (FSJ, circle) level functions.
Vector strength‐level function (VS‐LF) centered on the rate‐based threshold. The VS‐LF was fitted using a sigmoidal fit and the dynamic range, displayed by the black line above the VS‐LF, defined as the level difference yielding a driven VS change equal to 90% of the maximum driving VS.

- A–D
Representative raster plots from acoustically (A, 300 µs acoustic click) and optogenetically (B, f‐Chrimson; C, vf‐Chrimson; D, vf‐Chrimson‐ES/TS; 1 ms light pulse, λ = 594 nm) stimulated putative SGNs at different intensities: no click/light (SR), threshold (Thr), 50% and saturation (Sat).
- E–H
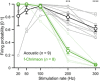
RLFs for acoustic (E, n = 19 SGNs, N = 3 mice) and optogenetic (F, f‐Chrimson, n = 14 SGNs, N = 2 mice; G, vf‐Chrimson, n = 26 SGNs, N = 2 mice; H, vf‐Chrimson‐ES/TS, n = 37 SGNs, N = 5 mice) SGN responses. Single RLFs are represented in gray; population RLFs were binned (bin width = 2 dB) and represented as average ± SEM. Population RLFs were fitted by a sigmoid function in order to extract the population dynamic range (reported in black above the population RLFs). The goodness of fit was expressed as R 2.
- I
Averaged threshold‐aligned acoustic and optogenetic RLFs (bin width= 2 dB, mean ± SEM) from the SGNs presented in (E‐H).
- J
Adaptation ratio (i.e., ratio between discharge rate measured from the first 100 ms and 350 ms) at threshold, mid‐intensity (50%), and saturated driven rate quantified from the SGNs presented in (E‐H), average ± SEM.
- K, L
Quantification of the acoustic and optogenetic RLFs dynamic range (J) and slope (K), same SGNs presented in (E‐H), computed from the first 100 ms (white fill) and 350 ms (colored fill) of the responses to click/light pulse trains. Boxes show 25th percentile, median, 75th percentile, and whiskers maximum and minimum.

- A–L
VS, FSL, and FSJ were computed from the first spike elicited by each click/light pulse. Single VS‐, FSL‐ and FSJ‐level functions, from the putative SGNs presented in Fig 5, were aligned on the rate‐based threshold and plotted in gray. Averaged (acoustic: bin = 5 dB; f‐Chrimson/vf‐Chrimson/vf‐Chrimson‐ES/TS: bin = 2 dB) level functions were plotted in color using the same color code than in Fig 5. Average ± SEM. Inserts in (I‐L) : Quantification of the dynamic range (10 – 90% of the difference between SR and saturation) quantified from the single VS‐level functions using a sigmoid fit. Boxes show 25th percentile, median, 75th percentile, and whiskers maximum and minimum. For acoustic, n = 19 SGNs, N = 3 mice; for f‐Chrimson, n = 14 SGNs, N = 2 mice; for vf‐Chrimson, n = 26 SGNs, N = 2 mice; for vf‐Chrimson‐ES/TS, n = 37 SGNs, N = 5 mice.

- A–L
Vector strength‐ (A‐D), FSL‐(E‐H), and FSJ‐ (I‐L) as a function of the discharge rate. The intensity above threshold (dB SL) is encoded by the color scale presented in (A‐D, acoustic: bin width = 6 dB; f‐Chrimson: bin width = 2.5 dB; vf‐Chrimson/vf‐Chrimson‐ES/TS: bin width = 1 dB). Mean ± SEM were plotted using as marker edge color, the color corresponding to the group and the intensity above threshold is indicated by the marker face color. For acoustic, n = 19 SGNs, N = 3 mice; for f‐Chrimson, n = 14 SGNs, N = 2 mice; for vf‐Chrimson, n = 26 SGNs, N = 2 mice; for vf‐Chrimson‐ES/TS, n = 37 SGNs, N = 5 mice.
References
-
- Bourien J, Tang Y, Batrel C, Huet A, Lenoir M, Ladrech S, Desmadryl G, Nouvian R, Puel J‐L, Wang J (2014) Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol 112: 1025–1039 - PubMed
-
- Busskamp V, Picaud S, Sahel JA, Roska B (2012) Optogenetic therapy for retinitis pigmentosa. Gene Ther 19: 169–175 - PubMed
-
- Cobeldick S (2018) ColorBrewer: attractive and distinctive colormaps MATLAB central file exchange
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

