Postmarketing Safety Monitoring After Influenza Vaccination Using a Mobile Health App: Prospective Longitudinal Feasibility Study
- PMID: 33960950
- PMCID: PMC8140379
- DOI: 10.2196/26289
Postmarketing Safety Monitoring After Influenza Vaccination Using a Mobile Health App: Prospective Longitudinal Feasibility Study
Abstract
Background: For the safety monitoring of vaccinations postlicensure, reports of adverse events after immunization (AEFIs) are crucial. New technologies such as digital mobile apps can be used as an active approach to capture these events. We therefore conducted a feasibility study among recipients of the influenza vaccination using an app for assessment of the reporting of AEFIs.
Objective: The goal of the research was to determine factors influencing adherence to and correct use of a newly developed app for individuals to report AEFI for 3 months using regular reminder functions, to identify determinants of AEFI occurrence and define reported AEFI types.
Methods: We developed the app (SafeVac) and offered it to recipients of the influenza vaccination in 3 occupational settings in fall 2018. In this prospective longitudinal feasibility study, data on AEFIs were generated through SafeVac for 3 months. Using logistic and Cox regression, we assessed associations between app adherence, correct app entry, AEFIs, and sociodemographic parameters.
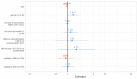
Results: Of the individuals who logged into SafeVac, 61.4% (207/337) used the app throughout a 3-month period. App use adherence was negatively associated with female sex (odds ratio [OR] 0.47; CI 0.25-0.91) and correct app entry was negatively associated with older age (OR 0.96; CI 0.93-0.99) and lower education (OR 0.31; CI 0.13-0.76). AEFI occurrence was associated with female sex (hazard ratio 1.41; CI 1.01-1.96) and negatively with older age (hazard ratio 0.98; CI 0.97-0.99). The most common AEFIs reported were injection site pain (106/337), pain in extremity (103/337), and fatigue/asthenia (73/337).
Conclusions: Digital AEFI reporting was feasible with SafeVac and generated plausible results for this observation period and setting. Studies directly comparing SafeVac with conventional passive reporting schemes could determine whether such digital approaches improve completeness, timeliness, and sensitivity of vaccine vigilance. Further studies should evaluate if these results are transferable to other vaccinations and populations and if introduction of such a tool has an influence on vaccination readiness and therefore vaccine safety.
Keywords: active reporting; adverse effect; adverse event; adverse event following immunization; digital health; mHealth; mobile health; pharmacovigilance; therapeutic use.
©Minh Tam H Nguyen, Gérard Krause, Brigitte Keller-Stanislawski, Stephan Glöckner, Dirk Mentzer, Jördis J Ott. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org), 07.05.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures





References
-
- Immunization coverage. Geneva: World Health Organization; [2021-04-06]. https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage.
-
- Vaccines. Geneva: World Health Organization; [2021-04-06]. https://www.who.int/topics/vaccines/en/
-
- Ständige Impfkommission (STIKO) beim Robert Koch-Institut [Ständige Impfkommission: Stellungnahme der Ständigen Impfkommission zu einer künftigen Impfung gegen COVID-19] Epid Bull. 2020;35:8–10. doi: 10.25646/7090. https://edoc.rki.de/bitstream/handle/176904/6936/ws20121_RKI_EB_35-2020_... - DOI
-
- Pharmacovigilance Risk Assessment Committee (PRAC) Interim guidance on enhanced safety surveillance for seasonal influenza vaccines in the EU. 2014. [2021-04-06]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin....
-
- Selecting viruses for the seasonal influenza vaccine. Atlanta: Centers for Disease Control and Prevention; [2021-04-06]. https://www.cdc.gov/flu/prevent/vaccine-selection.htm.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

