Extended Enolates: Versatile Intermediates for Asymmetric C-H Functionalization via Noncovalent Catalysis
- PMID: 33961323
- PMCID: PMC8361983
- DOI: 10.1002/chem.202100756
Extended Enolates: Versatile Intermediates for Asymmetric C-H Functionalization via Noncovalent Catalysis
Abstract
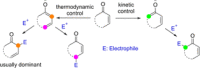
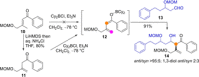
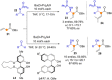
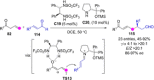
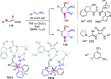
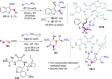
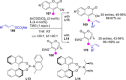
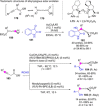
Catalyst-controlled functionalization of unmodified carbonyl compounds is a relevant operation in organic synthesis, especially when high levels of site- and stereoselectivity can be attained. This objective is now within reach for some subsets of enolizable substrates using various types of activation mechanisms. Recent contributions to this area include enantioselective transformations that proceed via transiently generated noncovalent di(tri)enolate-catalyst coordination species. While relatively easier to form than simple enolate congeners, di(tri)enolates are ambifunctional in nature and so control of the reaction regioselectivity becomes an issue. This Minireview discusses in some detail this and other problems, and how noncovalent activation approaches based on metallic and metal free catalysts have been developed to advance the field.
Keywords: Brønsted base catalysis; C−H functionalization; asymmetric catalysis; dienolates; regioselectivity.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


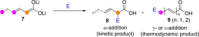










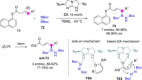
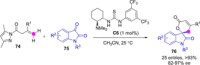
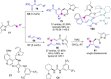
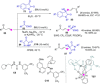



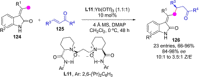









References
-
- Braun M., Modern Enolate Chemistry: From Preparation to Applications in Asymmetric Synthesis, Wiley-VCH, Weinheim, Germany, 2015.
-
- None
-
- Mukaiyama T., Narasaka K., Banno K., Chem. Lett. 1973, 1011–1014. First enantioselective version:
-
- Mukaiyama T., Kobayashi S., Uchiro H., Shiina I., Chem. Lett. 1990, 129–132.
-
- For selected reviews on Mukaiyama aldol reaction, see:
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

