Variants in the degron of AFF3 are associated with intellectual disability, mesomelic dysplasia, horseshoe kidney, and epileptic encephalopathy
- PMID: 33961779
- PMCID: PMC8206167
- DOI: 10.1016/j.ajhg.2021.04.001
Variants in the degron of AFF3 are associated with intellectual disability, mesomelic dysplasia, horseshoe kidney, and epileptic encephalopathy
Abstract
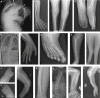
The ALF transcription factor paralogs, AFF1, AFF2, AFF3, and AFF4, are components of the transcriptional super elongation complex that regulates expression of genes involved in neurogenesis and development. We describe an autosomal dominant disorder associated with de novo missense variants in the degron of AFF3, a nine amino acid sequence important for its binding to ubiquitin ligase, or with de novo deletions of this region. The sixteen affected individuals we identified, along with two previously reported individuals, present with a recognizable pattern of anomalies, which we named KINSSHIP syndrome (KI for horseshoe kidney, NS for Nievergelt/Savarirayan type of mesomelic dysplasia, S for seizures, H for hypertrichosis, I for intellectual disability, and P for pulmonary involvement), partially overlapping the AFF4-associated CHOPS syndrome. Whereas homozygous Aff3 knockout mice display skeletal anomalies, kidney defects, brain malformations, and neurological anomalies, knockin animals modeling one of the microdeletions and the most common of the missense variants identified in affected individuals presented with lower mesomelic limb deformities like KINSSHIP-affected individuals and early lethality, respectively. Overexpression of AFF3 in zebrafish resulted in body axis anomalies, providing some support for the pathological effect of increased amount of AFF3. The only partial phenotypic overlap of AFF3- and AFF4-associated syndromes and the previously published transcriptome analyses of ALF transcription factors suggest that these factors are not redundant and each contributes uniquely to proper development.
Keywords: AFF3; AFF4; horseshoe kidney; intellectual disability; mesomelic dysplasia.
Copyright © 2021 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
T.F., G.D., J.J., L.R., and R.E.S. and W.K.C. are employees and former employees of GeneDx, respectively. The remaining authors declare no competing interests.
Figures





References
-
- Bitoun E., Davies K.E. The robotic mouse: unravelling the function of AF4 in the cerebellum. Cerebellum. 2005;4:250–260. - PubMed
-
- Nilson I., Reichel M., Ennas M.G., Greim R., Knörr C., Siegler G., Greil J., Fey G.H., Marschalek R. Exon/intron structure of the human AF-4 gene, a member of the AF-4/LAF-4/FMR-2 gene family coding for a nuclear protein with structural alterations in acute leukaemia. Br. J. Haematol. 1997;98:157–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

