Extracellular vesicles regulate gap junction-mediated intercellular communication and HIV-1 infection of human neural progenitor cells
- PMID: 33962010
- PMCID: PMC9056881
- DOI: 10.1016/j.nbd.2021.105388
Extracellular vesicles regulate gap junction-mediated intercellular communication and HIV-1 infection of human neural progenitor cells
Abstract
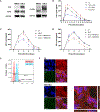
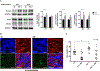
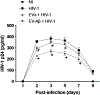
Human immunodeficiency virus-1 (HIV-1) has been shown to cross the blood-brain barrier and cause HIV-associated neurocognitive disorders (HAND) through a process that may involve direct or indirect interactions with the central nervous system (CNS) cells and alterations of amyloid β (Aβ) homeostasis. The present study focused on the mechanisms of HIV-1 infecting human neural progenitor cells (hNPCs) and affecting NPC intercellular communications with human brain endothelial cells (HBMEC). Despite the lack of the CD4 receptor, hNPCs were effectively infected by HIV-1 via a mechanism involving the chemokine receptors, CXCR4 and CCR5. HIV-1 infection increased expression of connexin-43 (Cx43), phosphorylated Cx43 (pCx43), and pannexin 2 (Panx2) protein levels in hNPCs, suggesting alterations in gap-junction (GJ) and pannexin channel communication. Indeed, a functional GJ assay indicated an increase in communication between HIV-infected hNPCs and non-infected HBMEC. We next analyzed the impact of HBMEC-derived extracellular vesicles (EVs) and EVs carrying Aβ (EV-Aβ) on the expression of Cx43, pCx43, and Panx2 in HIV-1 infected and non-infected hNPCs. Exposure to EV-Aβ resulted in significant reduction of Cx43 and pCx43 protein expression in non-infected hNPCs when compared to EV controls. Interestingly, EV-Aβ treatment significantly increased levels of Cx43, pCx43, and Panx2 in HIV-1-infected hNPCs when compared to non-infected controls. These results were confirmed in a GJ functional assay and an ATP release assay, which is an indicator of connexin hemichannel and/or pannexin channel functions. Overall, the current study demonstrates the importance of hNPCs in HIV-1 infection and indicates that intercellular communications between infected hNPCs and HBMEC can be effectively modulated by EVs carrying Aβ as their cargo.
Keywords: Amyloid β; Connexins; Extracellular vesicles; HIV-1; Human neural progenitor cells; Pannexins.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no conflict of interest.
Figures

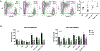
References
-
- Alvarez Losada S, Cantó-Nogués C, Muñoz-Fernández MA, 2002. A new possible mechanism of human immunodeficiency virus type 1 infection of neural cells. Neurobiol Dis. 11, 469–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

