Insulin-like growth factors: Ligands, binding proteins, and receptors
- PMID: 33962049
- PMCID: PMC8513159
- DOI: 10.1016/j.molmet.2021.101245
Insulin-like growth factors: Ligands, binding proteins, and receptors
Abstract
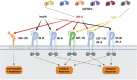
Background: The insulin-like growth factor family of ligands (IGF-I, IGF-II, and insulin), receptors (IGF-IR, M6P/IGF-IIR, and insulin receptor [IR]), and IGF-binding proteins (IGFBP-1-6) play critical roles in normal human physiology and disease states.
Scope of review: Insulin and insulin receptors are the focus of other chapters in this series and will therefore not be discussed further. Here we review the basic components of the IGF system, their role in normal physiology and in critical pathology's. While this review concentrates on the role of IGFs in human physiology, animal models have been essential in providing understanding of the IGF system, and its regulation, and are briefly described.
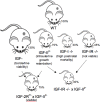
Major conclusions: IGF-I has effects via the circulation and locally within tissues to regulate cellular growth, differentiation, and survival, thereby controlling overall body growth. IGF-II levels are highest prenatally when it has important effects on growth. In adults, IGF-II plays important tissue-specific roles, including the maintenance of stem cell populations. Although the IGF-IR is closely related to the IR it has distinct physiological roles both on the cell surface and in the nucleus. The M6P/IGF-IIR, in contrast, is distinct and acts as a scavenger by mediating internalization and degradation of IGF-II. The IGFBPs bind IGF-I and IGF-II in the circulation to prolong their half-lives and modulate tissue access, thereby controlling IGF function. IGFBPs also have IGF ligand-independent cell effects.
Keywords: Cancer; Growth; Insulin-like growth factor binding proteins; Insulin-like growth factor receptors; Insulin-like growth factors; Metabolism.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Salmon W.D., Jr., Daughaday W.H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. The Journal of Laboratory and Clinical Medicine. 1957;49(6):825–836. - PubMed
-
- Powell-Braxton L., Hollingshead P., Warburton C., Dowd M., Pitts-Meek S., Dalton D. IGF-I is required for normal embryonic growth in mice. Genes & Development. 1993;7(12B):2609–2617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

