A Comparative Study of Real-Time RT-PCR-Based SARS-CoV-2 Detection Methods and Its Application to Human-Derived and Surface Swabbed Material
- PMID: 33962053
- PMCID: PMC8096526
- DOI: 10.1016/j.jmoldx.2021.04.009
A Comparative Study of Real-Time RT-PCR-Based SARS-CoV-2 Detection Methods and Its Application to Human-Derived and Surface Swabbed Material
Abstract
Real-time RT-PCR remains a gold standard in the detection of various viral diseases. In the coronavirus 2019 pandemic, multiple RT-PCR-based tests were developed to screen for viral infection. As an emergency response to increasing testing demand, we established a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR diagnostics platform for which we compared different commercial and in-house RT-PCR protocols. Four commercial, one customized, and one in-house RT-PCR protocols were evaluated with 92 SARS-CoV-2-positive and 92 SARS-CoV-2-negative samples. Furthermore, economical and practical characteristics of these protocols were compared. In addition, a highly sensitive digital droplet PCR (ddPCR) method was developed, and application of RT-PCR and ddPCR methods on SARS-CoV-2 environmental samples was examined. Very low limits of detection (1 or 2 viral copies/μL), high sensitivities (93.6% to 97.8%), and high specificities (98.7% to 100%) for the tested RT-PCR protocols were found. Furthermore, the feasibility of downscaling two of the commercial protocols, which could optimize testing capacity, was demonstrated. Tested commercial and customized RT-PCR detection kits show very good and comparable sensitivity and specificity, and the kits could be further optimized for use on SARS-CoV-2 viral samples derived from human and surface swabbed samples.
Copyright © 2021 Association for Molecular Pathology and American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
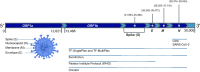

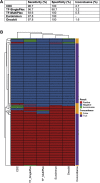

References
-
- Eichhoff O.M., Bellini E., Lienhard R., Stark W.J., Bechtold P., Grass R.N., Bosshard P.P., Levesque M.P. Comparison of RNA extraction methods for the detection of SARS-CoV-2 by RT-PCR. medRxiv. 2020 doi: 10.1101/2020.08.13.20172494. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

