Clinical and prognostic features of patients with detailed RAS/BRAF-mutant colorectal cancer in Japan
- PMID: 33962575
- PMCID: PMC8105976
- DOI: 10.1186/s12885-021-08271-z
Clinical and prognostic features of patients with detailed RAS/BRAF-mutant colorectal cancer in Japan
Abstract
Background: RAS/BRAFV600E mutations are the most remarkable oncogenic driver mutations in colorectal cancer (CRC) and play an important role in treatment selection. No data are available regarding the clinical and prognostic features of patients with detailed RAS/BRAFV600E-mutant metastatic CRC (mCRC) in Japan.
Methods: A total of 152 chemotherapy-naïve patients with mCRC were included in this study between August 2018 and July 2019. Tumor samples were collected, and RAS/BRAFV600E status was investigated. RAS/BRAFV600E status was examined using a MEBGEN RASKET-B kit and polymerase chain reaction reverse sequence-specific oligonucleotide method.
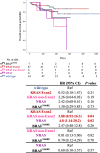
Results: RAS/BRAFV600E mutations were detected in 54% of cases (KRAS codon 12, 26%; KRAS codon 13, 17%; KRAS non-Exon2, 5%; NRAS, 5%; and BRAFV600E, 7%). BRAFV600E-mutant CRC mainly existed in the right colon, whereas KRAS non-Exon2 and NRAS-mutant CRC was predominantly present in the left colon. KRAS non-Exon2 and NRAS-mutant CRC were associated with shorter survival time than RAS wild-type CRC (hazard ratio [HR], 2.26; 95% confidence interval [CI], 0.64-8.03; p = 0.19; HR, 2.42; 95% CI, 0.68-8.61; p = 0.16) and significantly shorter overall survival than KRAS Exon2-mutant CRC (HR, 3.88; 95% CI, 0.92-16.3; p = 0.04; HR, 4.80; 95% CI, 1.14-20.2; p = 0.02).
Conclusions: In our multicenter study, the findings elucidated the clinical and prognostic features of patients with detailed RAS/BRAFV600E-mutant mCRC in Japan.
Keywords: Colorectal cancer; KRAS Exon2; KRAS non-Exon2; NRAS.
Conflict of interest statement
HS has received research funding from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and honoraria from Bayer, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Eli Lilly Japan, Merck Bio Pharma, MSD, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical Co., Ltd., Takeda, and Yakult Honsha.
MK has received honoraria from Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Yakult, Taiho Pharma, and Merck Biopharma Co., Ltd.
The other physicians have no COI.
Figures




References
-
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. New Engl J Med. 2013;369(11):1023–1034. doi: 10.1056/NEJMoa1305275. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

