Developmental up-regulation of NMDA receptors in the prefrontal cortex and hippocampus of mGlu5 receptor knock-out mice
- PMID: 33962661
- PMCID: PMC8106212
- DOI: 10.1186/s13041-021-00784-9
Developmental up-regulation of NMDA receptors in the prefrontal cortex and hippocampus of mGlu5 receptor knock-out mice
Erratum in
-
Correction to: Developmental up-regulation of NMDA receptors in the prefrontal cortex and hippocampus of mGlu5 receptor knock-out mice.Mol Brain. 2021 Jul 16;14(1):114. doi: 10.1186/s13041-021-00822-6. Mol Brain. 2021. PMID: 34271968 Free PMC article. No abstract available.
Abstract
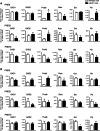
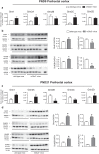
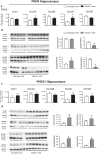
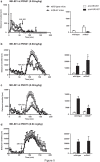
mGlu5 metabotropic glutamate receptors are highly expressed and functional in the early postnatal life, and are known to positively modulate NMDA receptor function. Here, we examined the expression of NMDA receptor subunits and interneuron-related genes in the prefrontal cortex and hippocampus of mGlu5-/- mice and wild-type littermates at three developmental time points (PND9, - 21, and - 75). We were surprised to find that expression of all NMDA receptor subunits was greatly enhanced in mGlu5-/- mice at PND21. In contrast, at PND9, expression of the GluN2B subunit was enhanced, whereas expression of GluN2A and GluN2D subunits was reduced in both regions. These modifications were transient and disappeared in the adult life (PND75). Changes in the transcripts of interneuron-related genes (encoding parvalbumin, somatostatin, vasoactive intestinal peptide, reelin, and the two isoforms of glutamate decarboxylase) were also observed in mGlu5-/- mice across postnatal development. For example, the transcript encoding parvalbumin was up-regulated in the prefrontal cortex of mGlu5-/- mice at PND9 and PND21, whereas it was significantly reduced at PND75. These findings suggest that in mGlu5-/- mice a transient overexpression of NMDA receptor subunits may compensate for the lack of the NMDA receptor partner, mGlu5. Interestingly, in mGlu5-/- mice the behavioral response to the NMDA channel blocker, MK-801, was significantly increased at PND21, and largely reduced at PND75. The impact of adaptive changes in the expression of NMDA receptor subunits should be taken into account when mGlu5-/- mice are used for developmental studies.
Keywords: Hippocampus MK-801; Interneuron related genes; Locomotor activity; NMDA receptor subunits; Prefrontal cortex.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

Similar articles
-
Altered expression of schizophrenia-related genes in mice lacking mGlu5 receptors.Eur Arch Psychiatry Clin Neurosci. 2018 Feb;268(1):77-87. doi: 10.1007/s00406-016-0728-z. Epub 2016 Aug 31. Eur Arch Psychiatry Clin Neurosci. 2018. PMID: 27581816
-
Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release.Neuropsychopharmacology. 2004 Jul;29(7):1259-69. doi: 10.1038/sj.npp.1300417. Neuropsychopharmacology. 2004. PMID: 15010696
-
Dizocilpine (MK-801) induces distinct changes of N-methyl-D-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex.Int J Neuropsychopharmacol. 2009 Nov;12(10):1395-408. doi: 10.1017/S146114570900042X. Epub 2009 May 13. Int J Neuropsychopharmacol. 2009. PMID: 19435549 Free PMC article.
-
Tonic Activation of GluN2C/GluN2D-Containing NMDA Receptors by Ambient Glutamate Facilitates Cortical Interneuron Maturation.J Neurosci. 2019 May 8;39(19):3611-3626. doi: 10.1523/JNEUROSCI.1392-18.2019. Epub 2019 Mar 7. J Neurosci. 2019. PMID: 30846615 Free PMC article.
-
Interneuron NMDA Receptor Ablation Induces Hippocampus-Prefrontal Cortex Functional Hypoconnectivity after Adolescence in a Mouse Model of Schizophrenia.J Neurosci. 2020 Apr 15;40(16):3304-3317. doi: 10.1523/JNEUROSCI.1897-19.2020. Epub 2020 Mar 23. J Neurosci. 2020. PMID: 32205341 Free PMC article.
Cited by
-
Early-life stress differentially affects CA3 synaptic inputs converging on apical and basal dendrites of CA1 pyramidal neurons.Front Neural Circuits. 2025 Feb 19;19:1533791. doi: 10.3389/fncir.2025.1533791. eCollection 2025. Front Neural Circuits. 2025. PMID: 40045983 Free PMC article.
-
Correction to: Developmental up-regulation of NMDA receptors in the prefrontal cortex and hippocampus of mGlu5 receptor knock-out mice.Mol Brain. 2021 Jul 16;14(1):114. doi: 10.1186/s13041-021-00822-6. Mol Brain. 2021. PMID: 34271968 Free PMC article. No abstract available.
References
-
- Nicoletti F, Wroblewski JT, Novelli A, Alho H, Guidotti A, Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. - DOI - PMC - PubMed
-
- Casabona G, Knöpfel T, Kuhn R, Gasparini F, Baumann P, Sortino MA, et al. Expression and coupling to polyphosphoinositide hydrolysis of group I metabotropic glutamate receptors in early postnatal and adult rat brain. Eur J Neurosci. 1997;9(1):12–17. doi: 10.1111/j.1460-9568.1997.tb01348.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

