Evaluation of two sexual-stage antigens as bivalent transmission-blocking vaccines in rodent malaria
- PMID: 33962671
- PMCID: PMC8103607
- DOI: 10.1186/s13071-021-04743-0
Evaluation of two sexual-stage antigens as bivalent transmission-blocking vaccines in rodent malaria
Abstract
Background: Transmission-blocking vaccine (TBV) is a promising strategy for malaria elimination. It is hypothesized that mixing or fusing two antigens targeting different stages of sexual development may provide higher transmission-blocking activity than these antigens used individually.
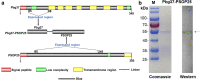
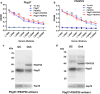
Methods: A chimeric protein composed of fragments of Pbg37 and PSOP25 was designed and expressed the recombinant protein in Escherichia coli Rosetta-gami B (DE3). After immunizing mice with individual recombinant proteins Pbg37 and PSOP25, mixed proteins (Pbg37+PSOP25), or the fusion protein (Pbg37-PSOP25), the antibody titers of individual sera were analyzed by ELISA. IFA and Western blot were performed to test the reactivity of the antisera with the native proteins in the parasite. The transmission-blocking activity of the different immunization schemes was assessed using in vitro and in vivo assays.
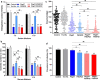
Results: When Pbg37 and PSOP25 were co-administered in a mixture or as a fusion protein, they elicited similar antibody responses in mice as single antigens without causing immunological interference with each other. Antibodies against the mixed or fused antigens recognized the target proteins in the gametocyte, gamete, zygote, and ookinete stages. The mixed proteins or the fusion protein induced antibodies with significantly stronger transmission-reducing activities in vitro and in vivo than individual antigens.
Conclusions: There was no immunological interference between Pbg37 and PSOP25. The bivalent vaccines, which expand the portion of the sexual development during which the transmission-blocking antibodies act, produced significantly stronger transmission-reducing activities than single antigens. Altogether, these data provide the theoretical basis for the development of combination TBVs targeting different sexual stages.
Keywords: Dual-antigen; Immunological interference; Transmission-blocking activity; Transmission-blocking vaccine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- WHO . World malaria report 2020. Geneva: World Health Organization; 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

