Allergy to COVID-19 vaccines: A current update
- PMID: 33962863
- PMCID: PMC8062405
- DOI: 10.1016/j.alit.2021.04.003
Allergy to COVID-19 vaccines: A current update
Abstract
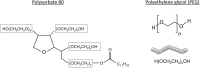
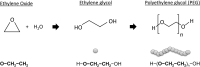
Adverse allergic reactions due to the administration of the vaccines developed for the protection of coronavirus disease 2019 (COVID-19) have been reported since the initiation of the vaccination campaigns. Current analyses provided by the Center for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) in the United States have estimated the rates of anaphylactic reactions in 2.5 and 11.1 per million of mRNA-1273 and BNT162b2 vaccines administered, respectively. Although rather low, such rates could have importance due to the uncommon fact that a large majority of the world population will be subjected to vaccination with the aforementioned vaccines in the following months and vaccination will most likely be necessary every season as for influenza vaccines. Health regulators have advised that any subject with a previous history of allergy to drugs or any component of the vaccines should not be vaccinated, however, certain misunderstanding exists since allergy to specific excipients in drugs and vaccines are in occasions misdiagnosed due to an absence of suspicion to specific excipients as allergenic triggers or due to inaccurate labeling or nomenclature. In this review, we provide an updated revision of the most current data regarding the anaphylactic reactions described for BNT162b2 vaccine, mRNA-1273 vaccine, and AZD1222 vaccine. We extensively describe the different excipients in the vaccines with the potential to elicit systemic allergic reactions such as polyethylene glycol (PEG), polysorbates, tromethamine/trometamol, and others and the possible immunological mechanisms involved.
Keywords: Anaphylaxis; COVID-19; SARS-CoV-2; Vaccine allergy; Vaccines.
Copyright © 2021 Japanese Society of Allergology. Production and hosting by Elsevier B.V. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

